This is a preprint.
A Futile Cycle?: Tissue Homeostatic Trans-Membrane Water Co-Transport: Kinetics, Thermodynamics, Metabolic Consequences
- PMID: 38659823
- PMCID: PMC11042311
- DOI: 10.1101/2024.04.17.589812
A Futile Cycle?: Tissue Homeostatic Trans-Membrane Water Co-Transport: Kinetics, Thermodynamics, Metabolic Consequences
Abstract
The phenomenon of active trans-membrane water cycling (AWC) has emerged in little over a decade. Here, we consider H2O transport across cell membranes from the origins of its study. Historically, trans-membrane water transport processes were classified into: A) compensating bidirectional fluxes ("exchange"), and B) unidirectional flux ("net flow") categories. Recent literature molecular structure determinations and molecular dynamic (MD) simulations indicate probably all the many different hydrophilic substrate membrane co-transporters have membrane-spanning hydrophilic pathways and co-transport water along with their substrates, and that they individually catalyze category A and/or B water flux processes, although usually not simultaneously. The AWC name signifies that, integrated over the all the cell's co-transporters, the rate of homeostatic, bidirectional trans-cytolemmal water exchange (category A) is synchronized with the metabolic rate of the crucial Na+,K+-ATPase (NKA) enzyme. A literature survey indicates the stoichiometric (category B) water/substrate ratios of individual co-transporters are often very large. The MD simulations also suggest how different co-transporter reactions can be kinetically coupled molecularly. Is this (Na+,K+-ATPase rate-synchronized) cycling futile, or is it consequential? Conservatively representative literature metabolomic and proteinomic results enable comprehensive free energy analyses of the many transport reactions with known water stoichiometries. Free energy calculations, using literature intracellular pressure (Pi) values reveals there is an outward trans-membrane H2O barochemical gradient of magnitude comparable to that of the well-known inward Na+ electrochemical gradient. For most co-influxers, these gradients are finely balanced to maintain intracellular metabolite concentration values near their consuming enzyme Michaelis constants. The thermodynamic analyses include glucose, glutamate-, gamma-aminobutyric acid (GABA), and lactate- transporters. 2%-4% Pi alterations can lead to disastrous concentration levels. For the neurotransmitters glutamate- and GABA, very small astrocytic Pi changes can allow/disallow synaptic transmission. Unlike the Na+ and K+ electrochemical steady-states, the H2O barochemical steady-state is in (or near) chemical equilibrium. The analyses show why the presence of aquaporins (AQPs) does not dissipate the trans-membrane pressure gradient. A feedback loop inherent in the opposing Na+ electrochemical and H2O barochemical gradients regulates AQP-catalyzed water flux as an integral AWC aspect. These results also require a re-consideration of the underlying nature of Pi. Active trans-membrane water cycling is not futile, but is inherent to the cell's "NKA system" - a new, fundamental aspect of biology.
Conflict of interest statement
CONFLICTS OF INTEREST: CSS and TMB are co-inventors on U.S. patent 11,728,038, “Activity MRI” (issued 15 August, 2023), which describes the MADI approach.
Figures



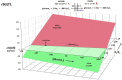



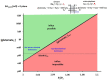
References
-
- Springer C. S., Baker E. M., Li X., Moloney B., Wilson G. J., Pike M. M., Barbara T. M., Rooney W. D., Maki J. H., Metabolic activity diffusion imaging [MADI]: I. Metabolic, cytometric modeling and simulations. NMR Biomed. 36, e4781 (2023). - PubMed
-
- Springer C. S., Baker E. M., Li X., Moloney B., Wilson G. J., Anderson V. C., Sammi M. K., Pike M. M., Garzotto M. G., Kopp R. P., Coakley F. V., Rooney W. D., Maki J. H., Metabolic activity diffusion imaging [MADI]: II. Non-invasive, high-resolution human brain imaging of sodium pump flux and cell metrics. NMR Biomed. 36, e4782 (2023). - PubMed
-
- Neil J. J., Ackerman J. J. H., Metabolic activity diffusion imaging (MADI): A new paradigm. NMR in Biomed. 36, e4841 (2023). - PubMed
-
- Schlegel J., Baker E., Holland S., Stoller J., Packwood W., Li X., Barajas R., Springer C., Pike M., Metabolic activity diffusion imaging [MADI] of rat brain glioma. Proc. Int. Soc. Magn. Reson. Med. 31, 3924 (2023).
-
- Pike M. M., Li X., Baetscher E., Barbara T. M., Sammi M. K., Stevens A. A., Springer C. S., Does MADI detect temporal brain metabolic activity changes?” Proc. Int. Soc. Magn. Reson. Med. 31, 5177 (2023).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
