This is a preprint.
Neurophysiological and Autonomic Dynamics of Threat Processing During Sustained Social Fear Generalization
- PMID: 38659834
- PMCID: PMC11042332
- DOI: 10.1101/2024.04.16.589830
Neurophysiological and Autonomic Dynamics of Threat Processing During Sustained Social Fear Generalization
Update in
-
Neurophysiological and Autonomic Dynamics of Threat Processing during Sustained Social Fear Generalization.J Cogn Neurosci. 2025 Feb 1;37(2):482-497. doi: 10.1162/jocn_a_02276. J Cogn Neurosci. 2025. PMID: 39536160
Abstract
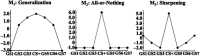
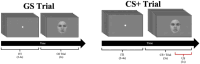
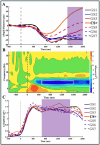
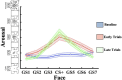
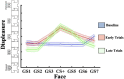
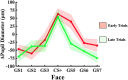
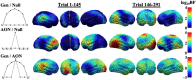
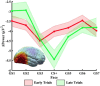
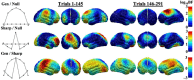
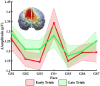
Survival in dynamic environments requires that organisms learn to predict danger from situational cues. One key facet of threat prediction is generalization from a predictive cue to similar cues, ensuring that a cue-outcome contingency is applied beyond the original learning environment. Generalization has been observed in laboratory studies of aversive conditioning: behavioral and physiological processes generalize responses from a stimulus paired with threat (the CS+) to unpaired stimuli, with response magnitudes varying with CS+ similarity. In contrast, work focusing on sensory responses in visual cortex has found a sharpening pattern, in which responses to stimuli closely resembling the CS+ are maximally suppressed, potentially reflecting lateral inhibitory interactions with the CS+ representation. Originally demonstrated with simple visual cues, changes in visuocortical tuning have also been observed in threat generalization learning across facial identities. It is unclear to what extent these visuocortical changes represent transient or sustained effects and if generalization learning requires prior conditioning to the CS+. The present study addressed these questions using EEG and pupillometry in an aversive generalization paradigm involving hundreds of trials using a gradient of facial identities. Visuocortical ssVEP sharpening occurred after dozens of trials of generalization learning without prior differential conditioning, but diminished as learning continued. By contrast, generalization of alpha power suppression, pupil dilation, and self-reported valence and arousal was seen throughout the experiment. Findings are consistent with threat processing models emphasizing the role of changing visucocortical and attentional dynamics when forming, curating, and shaping fear memories as observers continue learning about stimulus-outcome contingencies.
Figures

Similar articles
-
Neurophysiological and Autonomic Dynamics of Threat Processing during Sustained Social Fear Generalization.J Cogn Neurosci. 2025 Feb 1;37(2):482-497. doi: 10.1162/jocn_a_02276. J Cogn Neurosci. 2025. PMID: 39536160
-
Social aversive generalization learning sharpens the tuning of visuocortical neurons to facial identity cues.Elife. 2020 Jun 9;9:e55204. doi: 10.7554/eLife.55204. Elife. 2020. PMID: 32515731 Free PMC article.
-
Aversive Conditioning of Spatial Position Sharpens Neural Population-Level Tuning in Visual Cortex and Selectively Alters Alpha-Band Activity.J Neurosci. 2021 Jun 30;41(26):5723-5733. doi: 10.1523/JNEUROSCI.2889-20.2021. Epub 2021 May 25. J Neurosci. 2021. PMID: 34035136 Free PMC article.
-
Auditory aversive generalization learning prompts threat-specific changes in alpha-band activity.Cereb Cortex. 2024 Mar 1;34(3):bhae099. doi: 10.1093/cercor/bhae099. Cereb Cortex. 2024. PMID: 38517176 Free PMC article.
-
How fear conditioning affects the visuocortical processing of context cues in humans. Evidence from steady state visual evoked responses.Cortex. 2025 Feb;183:21-37. doi: 10.1016/j.cortex.2024.11.005. Epub 2024 Nov 19. Cortex. 2025. PMID: 39608048
References
-
- Adrian E. D., & Matthews B. H. (1934). The Berger rhythm: potential changes from the occipital lobes in man. Brain, 57(4), 355–385. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
