This is a preprint.
Loss of circulating CD8α+ NK cells during human Mycobacterium tuberculosis infection
- PMID: 38659858
- PMCID: PMC11042275
- DOI: 10.1101/2024.04.16.588542
Loss of circulating CD8α+ NK cells during human Mycobacterium tuberculosis infection
Abstract
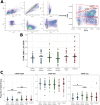
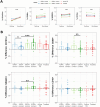
Natural Killer (NK) cells can recognize and kill Mtb-infected cells in vitro, however their role after natural human exposure has not been well-studied. To identify Mtb-responsive NK cell populations, we analyzed the peripheral blood of healthy household contacts of active Tuberculosis (TB) cases and source community donors in an endemic region of Port-au-Prince, Haiti by flow cytometry. We observed higher CD8α expression on NK cells in putative resistors (IGRA- contacts) with a progressive loss of these circulating cells during household-associated latent infection and disease. In vitro assays and CITE-seq analysis of CD8α+ NK cells demonstrated enhanced maturity, cytotoxic gene expression, and response to cytokine stimulation relative to CD8α- NK cells. CD8α+ NK cells also displayed dynamic surface expression dependent on MHC I in contrast to conventional CD8+ T cells. Together, these results support a specialized role for CD8α+ NK cell populations during Mtb infection correlating with disease resistance.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures





References
-
- Bagcchi S., WHO’s Global Tuberculosis Report 2022. Lancet Microbe 4, e20 (2023). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
