This is a preprint.
Geranylgeranylated-SCFFBXO10 Regulates Selective Outer Mitochondrial Membrane Proteostasis and Function
- PMID: 38659932
- PMCID: PMC11042265
- DOI: 10.1101/2024.04.16.589745
Geranylgeranylated-SCFFBXO10 Regulates Selective Outer Mitochondrial Membrane Proteostasis and Function
Update in
-
Geranylgeranylated SCFFBXO10 regulates selective outer mitochondrial membrane proteostasis and function.Cell Rep. 2024 Oct 22;43(10):114783. doi: 10.1016/j.celrep.2024.114783. Epub 2024 Sep 21. Cell Rep. 2024. PMID: 39306844 Free PMC article.
Abstract
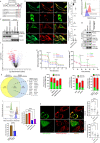
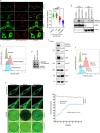
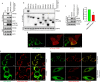
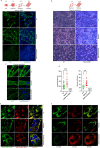
E3-ubiquitin ligases (E3s) are main components of the ubiquitin-proteasome system (UPS), as they determine substrate specificity in response to internal and external cues to regulate protein homeostasis. However, the regulation of membrane protein ubiquitination by E3s within distinct cell membrane compartments or organelles is not well understood. We show that FBXO10, the interchangeable component of the SKP1/CUL1/F-box ubiquitin ligase complex (SCF-E3), undergoes lipid-modification with geranylgeranyl isoprenoid at Cysteine953 (C953), facilitating its dynamic trafficking to the outer mitochondrial membrane (OMM). FBXO10 polypeptide does not contain a canonical mitochondrial targeting sequence (MTS); instead, its geranylgeranylation at C953 and the interaction with two cytosolic factors, PDE6δ (a prenyl group-binding protein), and HSP90 (a mitochondrial chaperone) orchestrate specific OMM targeting of prenyl-FBXO10 across diverse membrane compartments. The geranylgeranylation-deficient FBXO10(C953S) mutant redistributes away from the OMM, leading to impaired mitochondrial ATP production, decreased mitochondrial membrane potential, and increased mitochondrial fragmentation. Phosphoglycerate mutase 5 (PGAM5) was identified as a potential substrate of FBXO10 at the OMM using comparative quantitative mass spectrometry analyses of enriched mitochondria (LFQ-MS/MS), leveraging the redistribution of FBXO10(C953S). FBXO10, but not FBXO10(C953S), promoted polyubiquitylation and degradation of PGAM5. Examination of the role of this pathway in a physiological context revealed that the loss of FBXO10 or expression of prenylation-deficient-FBXO10(C953S) inhibited PGAM5 degradation, disrupted mitochondrial homeostasis, and impaired myogenic differentiation of human iPSCs and murine myoblasts. Our studies identify a mechanism for selective E3-ligase mediated regulation of mitochondrial membrane proteostasis and metabolic health, potentially amenable to therapeutic intervention.
Conflict of interest statement
Declaration of interests. None.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
