This is a preprint.
Activation of lysosomal iron triggers ferroptosis in cancer
- PMID: 38659936
- PMCID: PMC11042398
- DOI: 10.21203/rs.3.rs-4165774/v1
Activation of lysosomal iron triggers ferroptosis in cancer
Update in
-
Activation of lysosomal iron triggers ferroptosis in cancer.Nature. 2025 Jun;642(8067):492-500. doi: 10.1038/s41586-025-08974-4. Epub 2025 May 7. Nature. 2025. PMID: 40335696 Free PMC article.
Abstract
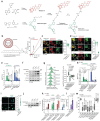
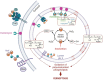
Iron catalyses the oxidation of lipids in biological membranes and promotes a form of cell death referred to as ferroptosis1-3. Identifying where this chemistry takes place in the cell can inform the design of drugs capable of inducing or inhibiting ferroptosis in various disease-relevant settings. Whereas genetic approaches have revealed underlying mechanisms of lipid peroxide detoxification1,4,5, small molecules can provide unparalleled spatiotemporal control of the chemistry at work6. Here, we show that the ferroptosis inhibitor liproxstatin-1 (Lip-1) exerts a protective activity by inactivating iron in lysosomes. Based on this, we designed the bifunctional compound fentomycin that targets phospholipids at the plasma membrane and activates iron in lysosomes upon endocytosis, promoting oxidative degradation of phospholipids and ferroptosis. Fentomycin effectively kills primary sarcoma and pancreatic ductal adenocarcinoma cells. It acts as a lipolysis-targeting chimera (LIPTAC), preferentially targeting iron-rich CD44high cell-subpopulations7,8 associated with the metastatic disease and drug resistance9,10. Furthermore, we demonstrate that fentomycin also depletes CD44high cells in vivo and reduces intranodal tumour growth in an immunocompetent murine model of breast cancer metastasis. These data demonstrate that lysosomal iron triggers ferroptosis and that lysosomal iron redox chemistry can be exploited for therapeutic benefits.
Conflict of interest statement
Additional Declarations: Yes there is potential Competing Interest. Institut Curie filed a patent application EP 24315029 related to LIPolysis-TArgeting Chimera to Induce Ferroptosis in Cancer. M.C. and B.P. hold patents for some of the compounds described herein, and are co-founders and shareholders of ROSCUE Therapeutics. B.R.S. is an inventor on patents and patent applications involving ferroptosis; co-founded and serves as a consultant to ProJenX, Inc. and Exarta Therapeutics; holds equity in Sonata Therapeutics; serves as a consultant to Weatherwax Biotechnologies Corporation and Akin Gump Strauss Hauer & Feld LLP.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

