This is a preprint.
Acute myeloid leukemia mitochondria hydrolyze ATP to resist chemotherapy
- PMID: 38659944
- PMCID: PMC11042215
- DOI: 10.1101/2024.04.12.589110
Acute myeloid leukemia mitochondria hydrolyze ATP to resist chemotherapy
Update in
-
Acute myeloid leukemia mitochondria hydrolyze ATP to support oxidative metabolism and resist chemotherapy.Sci Adv. 2025 Apr 11;11(15):eadu5511. doi: 10.1126/sciadv.adu5511. Epub 2025 Apr 9. Sci Adv. 2025. PMID: 40203117 Free PMC article.
Abstract
Despite early optimism, therapeutics targeting oxidative phosphorylation (OxPhos) have faced clinical setbacks, stemming from their inability to distinguish healthy from cancerous mitochondria. Herein, we describe an actionable bioenergetic mechanism unique to cancerous mitochondria inside acute myeloid leukemia (AML) cells. Unlike healthy cells which couple respiration to the synthesis of ATP, AML mitochondria were discovered to support inner membrane polarization by consuming ATP. Because matrix ATP consumption allows cells to survive bioenergetic stress, we hypothesized that AML cells may resist cell death induced by OxPhos damaging chemotherapy by reversing the ATP synthase reaction. In support of this, targeted inhibition of BCL-2 with venetoclax abolished OxPhos flux without impacting mitochondrial membrane potential. In surviving AML cells, sustained polarization of the mitochondrial inner membrane was dependent on matrix ATP consumption. Mitochondrial ATP consumption was further enhanced in AML cells made refractory to venetoclax, consequential to downregulations in both the proton-pumping respiratory complexes, as well as the endogenous F1-ATPase inhibitor ATP5IF1. In treatment-naive AML, ATP5IF1 knockdown was sufficient to drive venetoclax resistance, while ATP5IF1 overexpression impaired F1-ATPase activity and heightened sensitivity to venetoclax. Collectively, our data identify matrix ATP consumption as a cancer-cell intrinsic bioenergetic vulnerability actionable in the context of mitochondrial damaging chemotherapy.
Conflict of interest statement
ADS has received research funding from Takeda Pharmaceuticals and BMS, and consulting fees/honorarium from Takeda, Astra Zeneca, BMS and Novartis. ADS is named on a patent application for the use of DNT cells to treat AML. ADS is a member of the Medical and Scientific Advisory Board of the Leukemia and Lymphoma Society of Canada and the Therapy Acceleration Program for the Leukemia and Lymphoma Society. TPL has received Scientific Advisory Board membership, consultancy fees, honoraria, and/or stock options from Keystone Nano, Flagship Labs 86, Dren Bio, Recludix Pharma, Kymera Therapeutics, and Prime Genomics. DJF has received research funding, honoraria, and/or stock options from AstraZeneca, Dren Bio, Recludix Pharma, and Kymera Therapeutics.
Figures
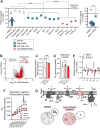


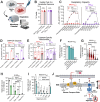
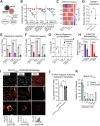



References
-
- Khan AUH, Rathore MG, Allende-Vega N, Vo DN, Belkhala S, Orecchioni S, Talarico G, Bertolini F, Cartron G, Lecellier CH, Villalba M. Human Leukemic Cells performing Oxidative Phosphorylation (OXPHOS) Generate an Antioxidant Response Independently of Reactive Oxygen species (ROS) Production. EBioMedicine. 2015. Nov 26;3:43–53. doi: 10.1016/j.ebiom.2015.11.045. - DOI - PMC - PubMed
-
- Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R, Bosc C, Sugita M, Stuani L, Fraisse M, Scotland S, Larrue C, Boutzen H, Féliu V, Nicolau-Travers ML, Cassant-Sourdy S, Broin N, David M, Serhan N, Sarry A, Tavitian S, Kaoma T, Vallar L, Iacovoni J, Linares LK, Montersino C, Castellano R, Griessinger E, Collette Y, Duchamp O, Barreira Y, Hirsch P, Palama T, Gales L, Delhommeau F, Garmy-Susini BH, Portais JC, Vergez F, Selak M, Danet-Desnoyers G, Carroll M, Récher C, Sarry JE. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017. Jul;7(7):716–735. doi: 10.1158/2159-8290.CD-16-0441. Epub 2017 Apr 17. - DOI - PMC - PubMed
-
- You R, Hou D, Wang B, Liu J, Wang X, Xiao Q, Pan Z, Li D, Feng X, Kang L, Chen P, Huang H. Bone marrow microenvironment drives AML cell OXPHOS addiction and AMPK inhibition to resist chemotherapy. J Leukoc Biol. 2022. Aug;112(2):299–311. doi: 10.1002/JLB.6A0821-409RR. Epub 2021 Dec 20. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
