Using SCENTinel® to predict SARS-CoV-2 infection: insights from a community sample during dominance of Delta and Omicron variants
- PMID: 38660364
- PMCID: PMC11041634
- DOI: 10.3389/fpubh.2024.1322797
Using SCENTinel® to predict SARS-CoV-2 infection: insights from a community sample during dominance of Delta and Omicron variants
Abstract
Introduction: Based on a large body of previous research suggesting that smell loss was a predictor of COVID-19, we investigated the ability of SCENTinel®, a newly validated rapid olfactory test that assesses odor detection, intensity, and identification, to predict SARS-CoV-2 infection in a community sample.
Methods: Between April 5, 2021, and July 5, 2022, 1,979 individuals took one SCENTinel® test, completed at least one physician-ordered SARS-CoV-2 PCR test, and endorsed a list of self-reported symptoms.
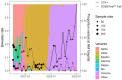
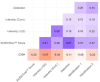
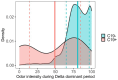
Results: Among the of SCENTinel® subtests, the self-rated odor intensity score, especially when dichotomized using a previously established threshold, was the strongest predictor of SARS-CoV-2 infection. SCENTinel® had high specificity and negative predictive value, indicating that those who passed SCENTinel® likely did not have a SARS-CoV-2 infection. Predictability of the SCENTinel® performance was stronger when the SARS-CoV-2 Delta variant was dominant rather than when the SARS-CoV-2 Omicron variant was dominant. Additionally, SCENTinel® predicted SARS-CoV-2 positivity better than using a self-reported symptom checklist alone.
Discussion: These results indicate that SCENTinel® is a rapid assessment tool that can be used for population-level screening to monitor abrupt changes in olfactory function, and to evaluate spread of viral infections like SARS-CoV-2 that often have smell loss as a symptom.
Keywords: COVID; anosmia; hyposmia; olfaction; pandemic; prediction; testing.
Copyright © 2024 Hunter, Zola, Ho, Kallen, Adjei-Danquah, Achenbach, Smith, Gershon, Reed, Schalet, Parma and Dalton.
Conflict of interest statement
On behalf of DR, VP, and PD, the Monell Chemical Senses Center and Temple University have been awarded patent protection (US patent no 11,337,640) and this patent has been licensed to Ahersla Health, Inc. DR, VP, and PD may benefit financially through their institution’s patent policy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
Cited by
-
Towards universal chemosensory testing: needs, barriers, and opportunities.Chem Senses. 2025 Jan 22;50:bjaf015. doi: 10.1093/chemse/bjaf015. Chem Senses. 2025. PMID: 40390292 Free PMC article.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

