Rare COVID-19 vaccine side effects got lost in the shuffle. Primary cutaneous lymphomas following COVID-19 vaccination: a systematic review
- PMID: 38660418
- PMCID: PMC11041019
- DOI: 10.3389/fmed.2024.1325478
Rare COVID-19 vaccine side effects got lost in the shuffle. Primary cutaneous lymphomas following COVID-19 vaccination: a systematic review
Abstract
Introduction: COVID-19 vaccines are generally safe and effective; however, they are associated with various vaccine-induced cutaneous side effects. Several reported cases of primary cutaneous lymphomas (CLs) following the COVID-19 vaccination have raised concerns about a possible association. This systematic review aims to investigate and elucidate the potential link between CLs and SARS-CoV-2 vaccines.
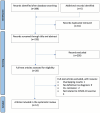
Methods: We performed a systematic literature search on PubMed, EBSCO and Scopus from January 01, 2019, to March 01, 2023, and analyzed studies based on determined eligibility criteria. The systematic review was performed based on the PRISMA protocol.
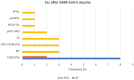
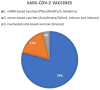
Results: A total of 12 articles (encompassing 24 patients) were included in this analysis. The majority of CLs were indolent cutaneous T-cell lymphomas (CTCLs) (66,7%; 16/24), with Lymphomatoid papulosis (LyP) being the most common type (33,3%; 8/24). Most patients (79,2%; 19/24) developed lesions after receiving the COVID-19 mRNA-based vaccines, and predominantly after the first immunization dose (54,2%; 13/24). The presented CLs cases exhibited a tendency to exacerbate following subsequent COVID-19 vaccinations. Nevertheless, CLs were characterized by a favorable course, leading to remission in most cases.
Conclusion: The available literature suggests an association between the occurrence and exacerbation of CLs with immune stimulation following COVID-19 vaccination. We hypothesize that post-vaccine CLs result from an interplay between cytokines and disrupted signaling pathways triggered by vaccine components, concurrently playing a pivotal role in the pathomechanism of CLs. However, establishing a definitive causal relationship between these events is currently challenging, primarily due to the relatively low rate of reported post-vaccine CLs. Nonetheless, these cases should not be disregarded, and patients with a history of lymphoproliferative disorders require post-COVID-19 vaccination monitoring to control the disease's course.Systematic review registrationwww.researchregistry.com, identifier [1723].
Keywords: COVID-19; SARS-CoV-2; SARS-CoV-2 mRNA vaccine; cutaneous lymphomas; side effects.
Copyright © 2024 Olszewska, Zaryczańska, Nowicki and Sokołowska-Wojdyło.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization Coronavirus Disease (COVID-19) Dashboard . (2024). Available at: https://covid19.who.int/ (Accessed January 21, 2024).
-
- World Health Association. COVID-19 advice for the public: Getting vaccinated . (2022). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19... (Accessed April 8, 2022)
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

