Unveiling Cryptosporidium parvum sporozoite-derived extracellular vesicles: profiling, origin, and protein composition
- PMID: 38660488
- PMCID: PMC11039866
- DOI: 10.3389/fcimb.2024.1367359
Unveiling Cryptosporidium parvum sporozoite-derived extracellular vesicles: profiling, origin, and protein composition
Abstract
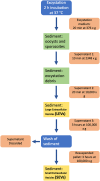
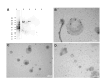
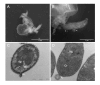
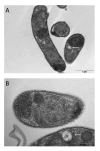
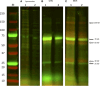
Cryptosporidium parvum is a common cause of a zoonotic disease and a main cause of diarrhea in newborns. Effective drugs or vaccines are still lacking. Oocyst is the infective form of the parasite; after its ingestion, the oocyst excysts and releases four sporozoites into the host intestine that rapidly attack the enterocytes. The membrane protein CpRom1 is a large rhomboid protease that is expressed by sporozoites and recognized as antigen by the host immune system. In this study, we observed the release of CpRom1 with extracellular vesicles (EVs) that was not previously described. To investigate this phenomenon, we isolated and resolved EVs from the excystation medium by differential ultracentrifugation. Fluorescence flow cytometry and transmission electron microscopy (TEM) experiments identified two types of sporozoite-derived vesicles: large extracellular vesicles (LEVs) and small extracellular vesicles (SEVs). Nanoparticle tracking analysis (NTA) revealed mode diameter of 181 nm for LEVs and 105 nm for SEVs, respectively. Immunodetection experiments proved the presence of CpRom1 and the Golgi protein CpGRASP in LEVs, while immune-electron microscopy trials demonstrated the localization of CpRom1 on the LEVs surface. TEM and scanning electron microscopy (SEM) showed that LEVs were generated by means of the budding of the outer membrane of sporozoites; conversely, the origin of SEVs remained uncertain. Distinct protein compositions were observed between LEVs and SEVs as evidenced by their corresponding electrophoretic profiles. Indeed, a dedicated proteomic analysis identified 5 and 16 proteins unique for LEVs and SEVs, respectively. Overall, 60 proteins were identified in the proteome of both types of vesicles and most of these proteins (48 in number) were already identified in the molecular cargo of extracellular vesicles from other organisms. Noteworthy, we identified 12 proteins unique to Cryptosporidium spp. and this last group included the immunodominant parasite antigen glycoprotein GP60, which is one of the most abundant proteins in both LEVs and SEVs.
Keywords: Cryptosporidium; GP60; aspartyl protease; excystation; exosome; extracellular vesicles; rhomboid; sporozoite.
Copyright © 2024 Bertuccini, Boussadia, Salzano, Vanni, Passerò, Nocita, Scaloni, Sanchez, Sargiacomo, Fiani and Tosini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Allison G. M., Rogers K. A., Borad A., Ahmed S., Karim M. M., Kane A. V., et al. . (2011). Antibody responses to the immunodominant Cryptosporidium gp15 antigen and gp15 polymorphisms in a case-control study of cryptosporidiosis in children in Bangladesh. Am. J. Trop. Med. Hyg. 85, 97–104. doi: 10.4269/ajtmh.2011.11-0043 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

