Activation of Pannexin-1 channels causes cell dysfunction and damage in mesangial cells derived from angiotensin II-exposed mice
- PMID: 38660621
- PMCID: PMC11041381
- DOI: 10.3389/fcell.2024.1387234
Activation of Pannexin-1 channels causes cell dysfunction and damage in mesangial cells derived from angiotensin II-exposed mice
Abstract
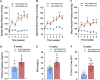
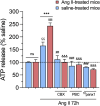
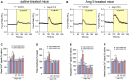
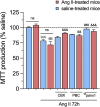
Chronic kidney disease (CKD) is a prevalent health concern associated with various pathological conditions, including hypertensive nephropathy. Mesangial cells are crucial in maintaining glomerular function, yet their involvement in CKD pathogenesis remains poorly understood. Recent evidence indicates that overactivation of Pannexin-1 (Panx1) channels could contribute to the pathogenesis and progression of various diseases. Although Panx1 is expressed in the kidney, its contribution to the dysfunction of renal cells during pathological conditions remains to be elucidated. This study aimed to investigate the impact of Panx1 channels on mesangial cell function in the context of hypertensive nephropathy. Using an Ang II-infused mouse model and primary mesangial cell cultures, we demonstrated that in vivo exposure to Ang II sensitizes cultured mesangial cells to show increased alterations when they are subjected to subsequent in vitro exposure to Ang II. Particularly, mesangial cell cultures treated with Ang II showed elevated activity of Panx1 channels and increased release of ATP. The latter was associated with enhanced basal intracellular Ca2+ ([Ca2+]i) and increased ATP-mediated [Ca2+]i responses. These effects were accompanied by increased lipid peroxidation and reduced cell viability. Crucially, all the adverse impacts evoked by Ang II were prevented by the blockade of Panx1 channels, underscoring their critical role in mediating cellular dysfunction in mesangial cells. By elucidating the mechanisms by which Ang II negatively impacts mesangial cell function, this study provides valuable insights into the pathogenesis of renal damage in hypertensive nephropathy.
Keywords: ATP; hypertensive nephropathy; inflammation; intracellular Ca2+; panx1.
Copyright © 2024 Lucero, Navarro, Barros-Osorio, Cáceres-Conejeros, Orellana and Gómez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Pannexin-1 Channels Are Essential for Mast Cell Degranulation Triggered During Type I Hypersensitivity Reactions.Front Immunol. 2019 Nov 29;10:2703. doi: 10.3389/fimmu.2019.02703. eCollection 2019. Front Immunol. 2019. PMID: 31849935 Free PMC article.
-
Angiotensin II-Induced Mesangial Cell Damaged Is Preceded by Cell Membrane Permeabilization Due to Upregulation of Non-Selective Channels.Int J Mol Sci. 2018 Mar 23;19(4):957. doi: 10.3390/ijms19040957. Int J Mol Sci. 2018. PMID: 29570626 Free PMC article.
-
Extracellular glucose reduces the responsiveness of mesangial cell ion channels to angiotensin II.Am J Physiol. 1995 Sep;269(3 Pt 2):F389-97. doi: 10.1152/ajprenal.1995.269.3.F389. Am J Physiol. 1995. PMID: 7573488
-
Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons.Int J Mol Sci. 2022 Dec 14;23(24):15936. doi: 10.3390/ijms232415936. Int J Mol Sci. 2022. PMID: 36555574 Free PMC article. Review.
-
Role of angiotensin II in diabetic nephropathy.Kidney Int Suppl. 2000 Sep;77:S93-8. doi: 10.1046/j.1523-1755.2000.07715.x. Kidney Int Suppl. 2000. PMID: 10997697 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

