SGLT2-independent effects of canagliflozin on NHE3 and mitochondrial complex I activity inhibit proximal tubule fluid transport and albumin uptake
- PMID: 38660713
- PMCID: PMC11381006
- DOI: 10.1152/ajprenal.00005.2024
SGLT2-independent effects of canagliflozin on NHE3 and mitochondrial complex I activity inhibit proximal tubule fluid transport and albumin uptake
Abstract
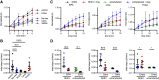
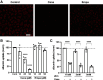
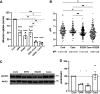
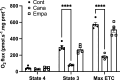
Beyond glycemic control, SGLT2 inhibitors (SGLT2is) have protective effects on cardiorenal function. Renoprotection has been suggested to involve inhibition of NHE3 leading to reduced ATP-dependent tubular workload and mitochondrial oxygen consumption. NHE3 activity is also important for regulation of endosomal pH, but the effects of SGLT2i on endocytosis are unknown. We used a highly differentiated cell culture model of proximal tubule (PT) cells to determine the direct effects of SGLT2i on Na+-dependent fluid transport and endocytic uptake in this nephron segment. Strikingly, canagliflozin but not empagliflozin reduced fluid transport across cell monolayers and dramatically inhibited endocytic uptake of albumin. These effects were independent of glucose and occurred at clinically relevant concentrations of drug. Canagliflozin acutely inhibited surface NHE3 activity, consistent with a direct effect, but did not affect endosomal pH or NHE3 phosphorylation. In addition, canagliflozin rapidly and selectively inhibited mitochondrial complex I activity. Inhibition of mitochondrial complex I by metformin recapitulated the effects of canagliflozin on endocytosis and fluid transport, whereas modulation of downstream effectors AMPK and mTOR did not. Mice given a single dose of canagliflozin excreted twice as much urine over 24 h compared with empagliflozin-treated mice despite similar water intake. We conclude that canagliflozin selectively suppresses Na+-dependent fluid transport and albumin uptake in PT cells via direct inhibition of NHE3 and of mitochondrial function upstream of the AMPK/mTOR axis. These additional targets of canagliflozin contribute significantly to reduced PT Na+-dependent fluid transport in vivo.NEW & NOTEWORTHY Reduced NHE3-mediated Na+ transport has been suggested to underlie the cardiorenal protection provided by SGLT2 inhibitors. We found that canagliflozin, but not empagliflozin, reduced NHE3-dependent fluid transport and endocytic uptake in cultured proximal tubule cells. These effects were independent of SGLT2 activity and resulted from inhibition of mitochondrial complex I and NHE3. Studies in mice are consistent with greater effects of canagliflozin versus empagliflozin on fluid transport. Our data suggest that these selective effects of canagliflozin contribute to reduced Na+-dependent transport in proximal tubule cells.
Keywords: metabolism; proximal tubule; sodium-glucose cotransporter; sodium/hydrogen exchanger.
Conflict of interest statement
O.A.W. serves as a consultant for Judo Biosciences. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures



Comment in
-
SGLT2 inhibitors: not every drug has the same effect.Am J Physiol Renal Physiol. 2024 Jun 1;326(6):F1039-F1040. doi: 10.1152/ajprenal.00126.2024. Epub 2024 May 2. Am J Physiol Renal Physiol. 2024. PMID: 38695073 No abstract available.
References
-
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. doi:10.1056/NEJMoa1811744. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- DK125049/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U54 DK137329/DK/NIDDK NIH HHS/United States
- S10 OD021627/OD/NIH HHS/United States
- P30 DK079307/DK/NIDDK NIH HHS/United States
- T32DK007052/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- T32 AG021885/AG/NIA NIH HHS/United States
- T32 DK061296/DK/NIDDK NIH HHS/United States
- R01 DK125049/DK/NIDDK NIH HHS/United States
- T32 DK007052/DK/NIDDK NIH HHS/United States
- T32AG021885/HHS | National Institutes of Health (NIH)
- S10OD021627/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- S10OD028596/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U54DK137329/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- predoctoral fellowship/American Society of Nephrology (ASN)
- S10 OD028596/OD/NIH HHS/United States
- T32DK061296/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- P30DK079307/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- MCB-2238694/NSF | Directorate for Biological Sciences (BIO)
LinkOut - more resources
Full Text Sources
Miscellaneous

