Trends in volumes and survival after hematopoietic cell transplantation in racial/ethnic minorities
- PMID: 38661372
- PMCID: PMC11260842
- DOI: 10.1182/bloodadvances.2023012469
Trends in volumes and survival after hematopoietic cell transplantation in racial/ethnic minorities
Abstract
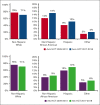
There has been an increase in volume as well as an improvement in overall survival (OS) after hematopoietic cell transplantation (HCT) for hematologic disorders. It is unknown if these changes have affected racial/ethnic minorities equally. In this observational study from the Center for International Blood and Marrow Transplant Research of 79 904 autologous (auto) and 65 662 allogeneic (allo) HCTs, we examined the volume and rates of change of autoHCT and alloHCT over time and trends in OS in 4 racial/ethnic groups: non-Hispanic Whites (NHWs), non-Hispanic African Americans (NHAAs), and Hispanics across 5 2-year cohorts from 2009 to 2018. Rates of change were compared using Poisson model. Adjusted and unadjusted Cox proportional hazards models examined trends in mortality in the 4 racial/ethnic groups over 5 study time periods. The rates of increase in volume were significantly higher for Hispanics and NHAAs vs NHW for both autoHCT and alloHCT. Adjusted overall mortality after autoHCT was comparable across all racial/ethnic groups. NHAA adults (hazard ratio [HR] 1.13; 95% confidence interval [CI] 1.04-1.22; P = .004) and pediatric patients (HR 1.62; 95% CI 1.3-2.03; P < .001) had a higher risk of mortality after alloHCT than NHWs. Improvement in OS over time was seen in all 4 groups after both autoHCT and alloHCT. Our study shows the rate of change for the use of autoHCT and alloHCT is higher in NHAAs and Hispanics than in NHWs. Survival after autoHCT and alloHCT improved over time; however, NHAAs have worse OS after alloHCT, which has persisted. Continued efforts are needed to mitigate disparities for patients requiring alloHCT.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.A. reports consultancy for GlaxoSmithKline (GSK), Sanofi, Bristol Myers Squibb (BMS), Takeda, BeiGene, Janssen, Regeneron, Cellectar, and Pfizer, and research funding from GSK, BMS, Pharmacyclics, Amgen, Janssen, Cellectar Biosciences, AbbVie, Ascentage, and Sanofi. C.U. reports honoraria (speakers’ bureau) from Takeda. S.C. reports advisory board participation for Hansa Therapeutics, CareDx, Spectrum/Acrotech Biopharma, Kiadis Pharma, Allogene, Celularity, MolMed, and Pharmacyclics, and research funding from Miltenyi Biotech and Kiadis Pharma. B.N.S. reports a government conflict-of-interest. A. Sharma reports compensation (consulting) for Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, and Editas Medicine; serving as a medical monitor for RCI BMT CSIDE clinical trial that receives financial compensation; research funding from CRISPR Therapeutics; honoraria from Vindico Medical Education; serving as the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A. Sharma’s institution. A. Sharma has no direct financial interest in these therapies. J.C. reports being a data safety and monitoring board member for ICON-AlloVir Inc, and ICON-Prolacta; being an advisory board member for MERIT CRO, and BMS; and stocks for Actinium Pharmaceuticals Inc, bluebird bio, Cellectar Biosciences, Dynavax Technologies, Gamida Cell, aTyr Pharma, Novavax, Ovid Therapeutics, Sorrento Therapeutics, VERY Inc, Viridian Therapeutics, Vaxart Inc, and 2seventy bio Inc, and Reg SHS. A.H.K. reports research funding from CareDx. T.K.-K. reports consulting fees (data safety monitoring committee) from Orca Bio. A. Steinberg reports speakers’ bureau participation for Jazz Pharmaceuticals, and advisory board participation for MorphoSys. W.A.W. reports research funding (to institution) from Pfizer and Genentech; serves as an adviser for Koneksa Health; and consulting for Teladoc. H.G.R. reports being a medical monitor for the CD33 chimeric antigen receptor study (unpaid), and having a contract with National Marrow Donor Program (unpaid). The remaining authors declare no competing financial interests.
Figures


References
-
- Auletta JJ KJ, Chen M, Shaw BE. Current Use and Outcome of Hematopoietic Stem Cell Transplantation: CIBMTR US Summary Slides. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages... CIBMTR.
-
- McCarthy PL, Jr., Hahn T, Hassebroek A, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995-2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19(7):1116–1123. - PMC - PubMed

