Do high-protein diets have the potential to reduce gut barrier function in a sex-dependent manner?
- PMID: 38662018
- PMCID: PMC11377480
- DOI: 10.1007/s00394-024-03407-w
Do high-protein diets have the potential to reduce gut barrier function in a sex-dependent manner?
Abstract
Purpose: Impaired gut barrier function is associated with systemic inflammation and many chronic diseases. Undigested dietary proteins are fermented in the colon by the gut microbiota which produces nitrogenous metabolites shown to reduce barrier function in vitro. With growing evidence of sex-based differences in gut microbiotas, we determined whether there were sex by dietary protein interactions which could differentially impact barrier function via microbiota modification.
Methods: Fermentation systems were inoculated with faeces from healthy males (n = 5) and females (n = 5) and supplemented with 0.9 g of non-hydrolysed proteins sourced from whey, fish, milk, soya, egg, pea, or mycoprotein. Microbial populations were quantified using fluorescence in situ hybridisation with flow cytometry. Metabolite concentrations were analysed using gas chromatography, solid phase microextraction coupled with gas chromatography-mass spectrometry and ELISA.
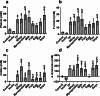
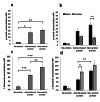
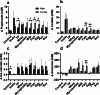
Results: Increased protein availability resulted in increased proteolytic Bacteroides spp (p < 0.01) and Clostridium coccoides (p < 0.01), along with increased phenol (p < 0.01), p-cresol (p < 0.01), indole (p = 0.018) and ammonia (p < 0.01), varying by protein type. Counts of Clostridium cluster IX (p = 0.03) and concentration of p-cresol (p = 0.025) increased in males, while females produced more ammonia (p = 0.02), irrespective of protein type. Further, we observed significant sex-protein interactions affecting bacterial populations and metabolites (p < 0.005).
Conclusions: Our findings suggest that protein fermentation by the gut microbiota in vitro is influenced by both protein source and the donor's sex. Should these results be confirmed through human studies, they could have major implications for developing dietary recommendations tailored by sex to prevent chronic illnesses.
Keywords: Dietary protein; Gut microbiota; In vitro gut systems; Microbial-derived metabolic end products; Sexual dimorphisms.
© 2024. The Author(s).
Conflict of interest statement
One of the authors, John Gibson, works for Food and Feed Innovations which partly funded this research.
Figures






Similar articles
-
Prebiotics Inhibit Proteolysis by Gut Bacteria in a Host Diet-Dependent Manner: a Three-Stage Continuous In Vitro Gut Model Experiment.Appl Environ Microbiol. 2020 May 5;86(10):e02730-19. doi: 10.1128/AEM.02730-19. Print 2020 May 5. Appl Environ Microbiol. 2020. PMID: 32198169 Free PMC article.
-
Prebiotic Supplementation of In Vitro Fecal Fermentations Inhibits Proteolysis by Gut Bacteria, and Host Diet Shapes Gut Bacterial Metabolism and Response to Intervention.Appl Environ Microbiol. 2019 Apr 18;85(9):e02749-18. doi: 10.1128/AEM.02749-18. Print 2019 May 1. Appl Environ Microbiol. 2019. PMID: 30824442 Free PMC article.
-
Glycation of fish protein impacts its fermentation metabolites and gut microbiota during in vitro human colonic fermentation.Food Res Int. 2018 Nov;113:189-196. doi: 10.1016/j.foodres.2018.07.015. Epub 2018 Jul 6. Food Res Int. 2018. PMID: 30195513
-
Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health.Curr Protein Pept Sci. 2017;18(8):795-808. doi: 10.2174/1389203718666170216153505. Curr Protein Pept Sci. 2017. PMID: 28215168 Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
Cited by
-
In vitro fermentation potential of diet-derived fermentable proteins of thirty-one human foods.Br J Nutr. 2025 Jun 14;133(11):1456-1465. doi: 10.1017/S0007114525103541. Epub 2025 Jun 3. Br J Nutr. 2025. PMID: 40457807 Free PMC article.
References
-
- Gilbert JA et al (2011) Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis 21(Suppl 2):B16–31 - PubMed
-
- Smith EA, Macfarlane GT (1998) Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol Ecol 25(4):355–368
-
- Beaumont M et al (2017) Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans1. Am J Clin Nutr 106(4):1005–1019 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

