Home-Monitoring Vision Tests to Detect Active Neovascular Age-Related Macular Degeneration
- PMID: 38662399
- PMCID: PMC11046404
- DOI: 10.1001/jamaophthalmol.2024.0918
Home-Monitoring Vision Tests to Detect Active Neovascular Age-Related Macular Degeneration
Abstract
Importance: Most neovascular age-related macular degeneration (nAMD) treatments involve long-term follow-up of disease activity. Home-monitoring would reduce the burden on patients and their caregivers and release clinic capacity.
Objective: To evaluate 3 vision home-monitoring tests for patients to use to detect active nAMD compared with diagnosing active nAMD at hospital follow-up during the after-treatment monitoring phase.
Design, setting, and participants: This was a diagnostic test accuracy study wherein the reference standard was detection of active nAMD by an ophthalmologist at hospital follow-up. The 3 home-monitoring tests evaluated included the following: (1) the KeepSight Journal (KSJ [International Macular and Retinal Foundation]), which contains paper-based near-vision tests presented as word puzzles, (2) the MyVisionTrack (mVT [Genentech]) vision-monitoring mobile app, viewed on an Apple mobile operating system-based device, and (3) the MultiBit (MBT [Visumetrics]) app, viewed on an Apple mobile operating system-based device. Participants were asked to test weekly; mVT and MBT scores were transmitted automatically, and KSJ scores were returned to the research office every 6 months. Raw scores between hospital follow-ups were summarized as averages. Patients were recruited from 6 UK hospital eye clinics and were 50 years and older with at least 1 eye first treated for active nAMD for at least 6 months or longer to a maximum of 42 months before approach. Participants were stratified by time since starting treatment. Study data were analyzed from May to September 2021.
Exposures: The KSJ, mVT, and MBT were compared with the reference standard (in-hospital ophthalmologist examination).
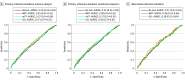
Main outcomes and measures: Estimated area under receiver operating characteristic curve (AUROC). The study had 90% power to detect a difference of 0.06, or 80% power to detect a difference of 0.05, if the AUROC for 2 tests was 0.75.
Results: A total of 297 patients (mean [SD] age, 74.9 [6.6] years; 174 female [58.6%]) were included in the study. At least 1 hospital follow-up was available for 312 study eyes in 259 participants (1549 complete visits). Median (IQR) home-monitoring testing frequency was 3 (1-4) times per month. Estimated AUROC was less than 0.6 for all home-monitoring tests, and only the KSJ summary score was associated with lesion activity (odds ratio, 3.48; 95% CI, 1.09-11.13; P = .04).
Conclusions and relevance: Results suggest that no home-monitoring vision test evaluated provided satisfactory diagnostic accuracy to identify active nAMD diagnosed in hospital eye service follow-up clinics. Implementing any of these evaluated tests, with ophthalmologists only reviewing test positives, would mean most active lesions were missed, risking unnecessary sight loss.
Conflict of interest statement
Figures

Comment on
-
More Homework for Patients With Macular Degeneration?JAMA Ophthalmol. 2024 Jun 1;142(6):520-521. doi: 10.1001/jamaophthalmol.2024.1050. JAMA Ophthalmol. 2024. PMID: 38662397 No abstract available.
References
-
- National Institute for Health and Care Excellence . Age-related macular degeneration. Accessed December 23, 2023. https://www.nice.org.uk/guidance/ng82 - PubMed
-
- Ying GS, Maguire MG, Daniel E, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group . Association of baseline characteristics and early vision response with 2-year vision outcomes in the comparison of AMD treatments trials (CATT). Ophthalmology. 2015;122(12):2523-31.e1. doi:10.1016/j.ophtha.2015.08.015 - DOI - PMC - PubMed

