Ciliopathy patient variants reveal organelle-specific functions for TUBB4B in axonemal microtubules
- PMID: 38662826
- PMCID: PMC7616230
- DOI: 10.1126/science.adf5489
Ciliopathy patient variants reveal organelle-specific functions for TUBB4B in axonemal microtubules
Erratum in
-
Erratum for the Research Article "Ciliopathy patient variants reveal organelle-specific functions for TUBB4B in axonemal microtubules," by Dodd et al.Science. 2024 May 10;384(6696):eadq2178. doi: 10.1126/science.adq2178. Epub 2024 May 9. Science. 2024. PMID: 38723101 No abstract available.
Abstract
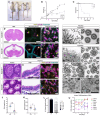
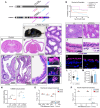
Tubulin, one of the most abundant cytoskeletal building blocks, has numerous isotypes in metazoans encoded by different conserved genes. Whether these distinct isotypes form cell type- and context-specific microtubule structures is poorly understood. Based on a cohort of 12 patients with primary ciliary dyskinesia as well as mouse mutants, we identified and characterized variants in the TUBB4B isotype that specifically perturbed centriole and cilium biogenesis. Distinct TUBB4B variants differentially affected microtubule dynamics and cilia formation in a dominant-negative manner. Structure-function studies revealed that different TUBB4B variants disrupted distinct tubulin interfaces, thereby enabling stratification of patients into three classes of ciliopathic diseases. These findings show that specific tubulin isotypes have distinct and nonredundant subcellular functions and establish a link between tubulinopathies and ciliopathies.
Conflict of interest statement
Figures




References
-
- Raff EC, Fackenthal JD, Hutchens JA, Hoyle HD, Turner FR. Microtubule architecture specified by a beta-tubulin isoform. Science. 1997;275:70–73. - PubMed
-
- Janke C, Magiera MM. The tubulin code and its role in controlling microtubule properties and functions. Nat Rev Mol Cell Biol. 2020;21:307–326. - PubMed
-
- Bahi-Buisson N, et al. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014;137:1676–1700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

