Safety and Dose-Response of Vidofludimus Calcium in Relapsing Multiple Sclerosis: Extended Results of a Placebo-Controlled Phase 2 Trial
- PMID: 38662979
- PMCID: PMC11087024
- DOI: 10.1212/NXI.0000000000200208
Safety and Dose-Response of Vidofludimus Calcium in Relapsing Multiple Sclerosis: Extended Results of a Placebo-Controlled Phase 2 Trial
Abstract
Background and objectives: Vidofludimus calcium suppressed MRI disease activity compared with placebo in patients with relapsing-remitting multiple sclerosis (RRMS) in the first cohort of the phase 2 EMPhASIS study. Because 30 mg and 45 mg showed comparable activity on multiple end points, the study enrolled an additional low-dose cohort to further investigate a dose-response relationship.
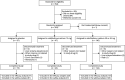
Methods: In a randomized, placebo-controlled, phase 2 trial, patients with RRMS, aged 18-55 years, and with ≥2 relapses in the last 2 years or ≥1 relapse in the last year, and ≥1 gadolinium-enhancing brain lesion in the last 6 months. Patients were randomly assigned (1:1:1) vidofludimus calcium (30 or 45 mg) or placebo in cohort 1 and vidofludimus calcium (10 mg) or placebo (4:1) in cohort 2 for 24 weeks. The primary end point was the cumulative number of combined unique active (CUA) lesions at week 24. Secondary end points were clinical outcomes and safety.
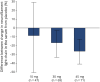
Results: Across cohorts 1 and 2, 268 patients were randomized to placebo (n = 81), 10 mg (n = 47) vidofludimus calcium, 30 mg (n = 71) vidofludimus calcium, or 45 mg (n = 69) vidofludimus calcium. The mean cumulative CUA lesions over 24 weeks was 5.8 (95% CI 4.1-8.2) for placebo, 5.9 (95% CI 3.9-9.0) for 10 mg treatment group, 1.4 (95% CI 0.9-2.1) for 30 mg treatment group, and 1.7 (95% CI 1.1-2.5) for 45 mg treatment group. Serum neurofilament light chain decreased in a dose-dependent manner. The number of patients with confirmed disability worsening after 24 weeks was 3 (3.7%) patients receiving placebo and 3 (1.6%) patients receiving any dose of vidofludimus calcium. Treatment-emergent adverse events occurred in 35 (43%) placebo patients compared with 11 (23%) and 71 (37%) patients in the 10 mg or any dose of vidofludimus calcium groups, respectively. The incidence of liver enzyme elevations and infections were similar between placebo and any dose of vidofludimus calcium. No new safety signals were observed.
Discussion: Compared with placebo, vidofludimus calcium suppressed the development of new brain lesions with daily doses of 30 mg and 45 mg, but not 10 mg, establishing the lowest efficacious dose is 30 mg.
Classification of evidence: This study provides Class II evidence that among adults with active RRMS and ≥1 Gd+ brain lesion in the past 6 months, the cumulative number of active lesions decreased with vidofludimus calcium.
Trial registration information: ClinicalTrials.gov (NCT03846219) and EudraCT (2018-001896-19).
Conflict of interest statement
R.J. Fox reports personal fees from AB Science, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Immunic AG, Janssen, Novartis, Sanofi, Siemens, and TG Therapeutics; clinical trial contracts from Biogen, Novartis, and Sanofi; H. Wiendl reports grants and personal fees from AbbVie, Biogen, Merck Serono, and Sanofi Genzyme; personal fees from Actelion, Alexion, Evgen, F. Hoffmann‐La Roche, Gemeinnützige Hertie‐reStiftung, Immunic, Lundbeck, MedDay Pharmaceuticals, Novartis, Roche Pharma AG, Teva, and WebMD Global; and grants from Deutsche Forschungsgesellschaft (DFG), Else Kröner Fresenius Foundation, European Union, Fresenius Foundation, German Ministry for Education and Research (BMBF), GlaxoSmithKline, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, NRW Ministry of Education and Research, PML Consortium, RE Children's Foundation, and Swiss MS Society, outside the submitted work; C. Wolf is a partner at Lycalis sprl and reports compensation for his organization from BMS, Celgene, Desitin, Immunic, Merck KGaA, Novartis, Roche, Synthon, Teva, and Viatris for consulting and speaking; N. De Stefano has received honoraria from Biogen‐Idec, Genzyme, Immunic, Merck, Novartis, Roche, Celgene, and Teva for consulting services, speaking, and travel support. He serves on advisory boards for Merck, Novartis, Biogen‐Idec, Immunic, Roche, and Genzyme, and he has received research grant support from the Italian MS Society; J. Sellner received honoraria for participation in advisory boards, consultancy, and lecturing from Biogen, BMS, Immunic, Merck, Novartis, Roche, and Sanofi; V. Gryb reports grants/personal fees from Medpace, PPD, PRA Health Sciences, PSI, Roche, Sanofi, and Verum; K. Rejdak is the president elect of the Polish Neurologic Society; P.S. Bozhinov reports no declaration of interests; D. Vitt is a shareholder and employee of trial sponsor and a holder of patents for the drug under investigation; H. Kohlhof is a shareholder and employee of trial sponsor and a holder of patents for the drug under investigation; M. Ondrus is an employee of trial sponsor; V. Sciacca is an employee of trial sponsor; A.R. Muehler is a shareholder and employee of trial sponsor and a holder of patents for the drug under investigation. Go to
Figures

References
-
- Fox RJ, Wiendl H, Wolf C, et al. A double-blind, randomized, placebo-controlled phase 2 trial evaluating the selective dihydroorotate dehydrogenase inhibitor vidofludimus calcium in relapsing-remitting multiple sclerosis. Ann Clin Transl Neurol. 2022;9(7):977-987. doi: 10.1002/ACN3.51574 - DOI - PMC - PubMed
-
- Cohen JA, Arnold DL, Comi G, et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):373-381. doi: 10.1016/S1474-4422(16)00018-1 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
