Generating Clinical-Grade Gene-Disease Validity Classifications Through the ClinGen Data Platforms
- PMID: 38663031
- PMCID: PMC12001867
- DOI: 10.1146/annurev-biodatasci-102423-112456
Generating Clinical-Grade Gene-Disease Validity Classifications Through the ClinGen Data Platforms
Abstract
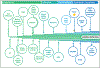
Clinical genetic laboratories must have access to clinically validated biomedical data for precision medicine. A lack of accessibility, normalized structure, and consistency in evaluation complicates interpretation of disease causality, resulting in confusion in assessing the clinical validity of genes and genetic variants for diagnosis. A key goal of the Clinical Genome Resource (ClinGen) is to fill the knowledge gap concerning the strength of evidence supporting the role of a gene in a monogenic disease, which is achieved through a process known as Gene-Disease Validity curation. Here we review the work of ClinGen in developing a curation infrastructure that supports the standardization, harmonization, and dissemination of Gene-Disease Validity data through the creation of frameworks and the utilization of common data standards. This infrastructure is based on several applications, including the ClinGen GeneTracker, Gene Curation Interface, Data Exchange, GeneGraph, and website.
Keywords: biocuration; clinical genetics; data harmonization; data standards; precision medicine; research informatics.
Figures




References
-
- Povey S, Lovering R, Bruford E, Wright M, Lush M, Wain H. 2001. The HUGO Gene Nomenclature Committee (HGNC). Hum. Genet 109:678–80 - PubMed
-
- Crespi S 2021. Looking back at 20 years of human genome sequencing. Science Podcast, Feb. 4. https://www.science.org/content/podcast/looking-back-20-years-human-geno...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

