Current advances in photocatalytic proximity labeling
- PMID: 38663396
- PMCID: PMC11193652
- DOI: 10.1016/j.chembiol.2024.03.012
Current advances in photocatalytic proximity labeling
Abstract
Understanding the intricate network of biomolecular interactions that govern cellular processes is a fundamental pursuit in biology. Over the past decade, photocatalytic proximity labeling has emerged as one of the most powerful and versatile techniques for studying these interactions as well as uncovering subcellular trafficking patterns, drug mechanisms of action, and basic cellular physiology. In this article, we review the basic principles, methodologies, and applications of photocatalytic proximity labeling as well as examine its modern development into currently available platforms. We also discuss recent key studies that have successfully leveraged these technologies and importantly highlight current challenges faced by the field. Together, this review seeks to underscore the potential of photocatalysis in proximity labeling for enhancing our understanding of cell biology while also providing perspective on technological advances needed for future discovery.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests D.W.C.M. declares an ownership interest in the company Dexterity Pharma LLC, which has commercialized materials used in this work. D.W.C.M. is an inventor on patents 20230100536 and 20220306683. D.W.C.M., S.D.K., and B.F.B. are inventors on provisional patent 63/428,899. D.W.C.M. and S.D.K. are inventors on provisional patent 63/419,519. D. W.C.M., S.D.K., and S.W.H. are inventors on provisional patent 63/424,581.
Figures


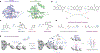


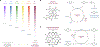
References
-
- Keskin O, Tuncbag N. & Gursoy A. Predicting protein–protein interactions from the molecular to the proteome level. Chemical reviews 2016, 116 (8), 4884–4909. - PubMed
-
- Bludau I. & Aebersold R. Proteomic and interactomic insights into the molecular basis of cell functional diversity. Nature Reviews Molecular Cell Biology 2020, 21 (6), 327–340. - PubMed
-
- Hentze MW, Castello A, Schwarzl T. & Preiss T. A brave new world of RNA-binding proteins. Nature reviews Molecular cell biology 2018, 19 (5), 327–341. - PubMed
-
- Casey JR, Grinstein S. & Orlowski J. Sensors and regulators of intracellular pH. Nature reviews Molecular cell biology 2010, 11 (1), 50–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

