Human anti-PSCA CAR macrophages possess potent antitumor activity against pancreatic cancer
- PMID: 38663406
- PMCID: PMC11162318
- DOI: 10.1016/j.stem.2024.03.018
Human anti-PSCA CAR macrophages possess potent antitumor activity against pancreatic cancer
Abstract
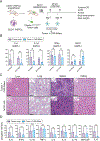
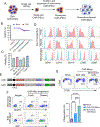
Due to the limitations of autologous chimeric antigen receptor (CAR)-T cells, alternative sources of cellular immunotherapy, including CAR macrophages, are emerging for solid tumors. Human induced pluripotent stem cells (iPSCs) offer an unlimited source for immune cell generation. Here, we develop human iPSC-derived CAR macrophages targeting prostate stem cell antigen (PSCA) (CAR-iMacs), which express membrane-bound interleukin (IL)-15 and truncated epidermal growth factor receptor (EGFR) for immune cell activation and a suicide switch, respectively. These allogeneic CAR-iMacs exhibit strong antitumor activity against human pancreatic solid tumors in vitro and in vivo, leading to reduced tumor burden and improved survival in a pancreatic cancer mouse model. CAR-iMacs appear safe and do not exhibit signs of cytokine release syndrome or other in vivo toxicities. We optimized the cryopreservation of CAR-iMac progenitors that remain functional upon thawing, providing an off-the-shelf, allogeneic cell product that can be developed into CAR-iMacs. Overall, our preclinical data strongly support the potential clinical translation of this human iPSC-derived platform for solid tumors, including pancreatic cancer.
Keywords: cancer immunotherapy; chimeric antigen receptor; cytokine release syndrome; human induced pluripotent stem cells; macrophages; off-the-shelf; pancreatic cancer; prostate stem cell antigen; solid tumor.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.Y., M.A.C., and Z.S. are in the process of a patent application at City of Hope.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

