Antifibrotic treatment adherence, efficacy and outcomes for patients with idiopathic pulmonary fibrosis in Spain: a real-world evidence study
- PMID: 38663886
- PMCID: PMC11043774
- DOI: 10.1136/bmjresp-2023-001687
Antifibrotic treatment adherence, efficacy and outcomes for patients with idiopathic pulmonary fibrosis in Spain: a real-world evidence study
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a rare disorder associated with increased mortality and morbidity. There are currently two drugs approved for IPF but their safety and efficacy profile in real-world settings in Spain is not well understood.
Methods: An observational, multicentre, prospective study was carried out among patients with IPF who started treatment with pirfenidone or nintedanib from 2015 to 2021. Data regarding clinical characteristics, drug adherence, safety profiles and clinical outcomes between these two drugs were collected.
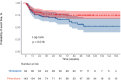
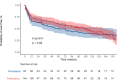
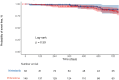
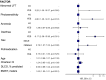
Results: 232 patients were included in the analysis. There were no meaningful differences between both groups at baseline. Patients who started pirfenidone showed a decreased risk for treatment withdrawal compared with those starting nintedanib (HR 0.65 (95% CI 0.46 to 0.94; p=0.002)). Time to first adverse event and all-cause mortality was similar between study groups. Risk factors for withdrawal were female sex, diarrhoea and photosensitivity.
Conclusions: in this real-world study, both pirfenidone and nintedanib showed similar efficacy profiles. Pirfenidone was associated with less treatment discontinuations due to side effects.
Keywords: interstitial fibrosis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BA reports grants and personal fees from GSK, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, non-financial support from Laboratorios Menarini, grants, personal fees and non-financial support from AstraZeneca, personal fees from Gilead, personal fees and non-financial support from MSD, personal fees from Laboratorios BIAL, personal fees from Zambon, outside the submitted work.
Figures
Similar articles
-
Pirfenidone vs. nintedanib in patients with idiopathic pulmonary fibrosis: a retrospective cohort study.Respir Res. 2021 Oct 19;22(1):268. doi: 10.1186/s12931-021-01857-y. Respir Res. 2021. PMID: 34666765 Free PMC article.
-
Incidence of acute exacerbation of idiopathic pulmonary fibrosis in patients receiving antifibrotic agents: Real-world experience.Respir Med. 2021 Oct;187:106551. doi: 10.1016/j.rmed.2021.106551. Epub 2021 Jul 26. Respir Med. 2021. PMID: 34343721
-
Comparative outcomes in patients receiving pirfenidone or nintedanib for idiopathic pulmonary fibrosis.Respir Res. 2021 May 4;22(1):135. doi: 10.1186/s12931-021-01714-y. Respir Res. 2021. PMID: 33947414 Free PMC article.
-
Real-world safety and effectiveness of pirfenidone and nintedanib in the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis.Eur J Clin Pharmacol. 2024 Oct;80(10):1445-1460. doi: 10.1007/s00228-024-03720-7. Epub 2024 Jul 4. Eur J Clin Pharmacol. 2024. PMID: 38963453
-
Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: a systematic review and meta-analysis.BMC Pulm Med. 2021 Dec 11;21(1):411. doi: 10.1186/s12890-021-01783-1. BMC Pulm Med. 2021. PMID: 34895203 Free PMC article.
Cited by
-
Safety, Tolerability, and Pharmacokinetics of SC1011 (Sufenidone), a Novel Antifibrotic Small Molecule, in Phase 1 Studies in Healthy Subjects.Clin Transl Sci. 2025 Mar;18(3):e70179. doi: 10.1111/cts.70179. Clin Transl Sci. 2025. PMID: 40065557 Free PMC article. Clinical Trial.
-
The Impact of Comorbidities on the Discontinuation of Antifibrotic Therapy in Patients with Idiopathic Pulmonary Fibrosis.Pharmaceuticals (Basel). 2025 Mar 14;18(3):411. doi: 10.3390/ph18030411. Pharmaceuticals (Basel). 2025. PMID: 40143187 Free PMC article.
-
Real-world treatment persistence and predictive factors for discontinuation of antifibrotic therapies in patients with idiopathic pulmonary fibrosis: a post-hoc analysis of two multicenter observational cohort studies in Poland.Front Pharmacol. 2025 Jun 5;16:1586197. doi: 10.3389/fphar.2025.1586197. eCollection 2025. Front Pharmacol. 2025. PMID: 40538535 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
