Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study
- PMID: 38663919
- PMCID: PMC11043905
- DOI: 10.1136/bmj-2023-078242
Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study
Erratum in
-
Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study.BMJ. 2024 May 16;385:q1094. doi: 10.1136/bmj.q1094. BMJ. 2024. PMID: 38754911 Free PMC article. No abstract available.
-
Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study.BMJ. 2024 Jun 5;385:q1237. doi: 10.1136/bmj.q1237. BMJ. 2024. PMID: 38839092 Free PMC article. No abstract available.
Abstract
Objective: To determine whether the combined use of glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 (SGLT-2) inhibitors is associated with a decreased risk of major adverse cardiovascular events and serious renal events compared with either drug class alone among patients with type 2 diabetes, and to assess the effect of the combination on the individual components of major adverse cardiovascular events, heart failure, and all cause mortality.
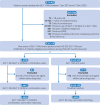
Design: Population based cohort study using a prevalent new-user design, emulating a trial.
Setting: UK Clinical Practice Research Datalink linked to Hospital Episode Statistics Admitted Patient Care and Office for National Statistics databases.
Participants: Two prevalent new-user cohorts were assembled between January 2013 and December 2020, with follow-up until the end of March 2021. The first cohort included 6696 patients who started GLP-1 receptor agonists and added on SGLT-2 inhibitors, and the second included 8942 patients who started SGLT-2 inhibitors and added on GLP-1 receptor agonists. Combination users were matched, in a 1:1 ratio, to patients prescribed the same background drug, duration of background drug, and time conditional propensity score.
Main outcome measures: Cox proportional hazards models were fitted to estimate the hazard ratios and 95% confidence intervals of major adverse cardiovascular events and serious renal events, separately, comparing the GLP-1 receptor agonist-SGLT-2 inhibitor combination with the background drug, either GLP-1 receptor agonists or SGLT-2 inhibitors, depending on the cohort. Secondary outcomes included associations with the individual components of major adverse cardiovascular events (myocardial infarction, ischaemic stroke, cardiovascular mortality), heart failure, and all cause mortality.
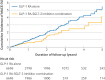
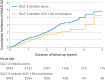
Results: Compared with GLP-1 receptor agonists, the SGLT-2 inhibitor-GLP-1 receptor agonist combination was associated with a 30% lower risk of major adverse cardiovascular events (7.0 v 10.3 events per 1000 person years; hazard ratio 0.70, 95% confidence interval 0.49 to 0.99) and a 57% lower risk of serious renal events (2.0 v 4.6 events per 1000 person years; hazard ratio 0.43, 0.23 to 0.80). Compared with SGLT-2 inhibitors, the GLP-1 receptor agonist-SGLT-2 inhibitor combination was associated with a 29% lower risk of major adverse cardiovascular events (7.6 v 10.7 events per 1000 person years; hazard ratio 0.71, 0.52 to 0.98), whereas serious renal events generated a wide confidence interval (1.4 v 2.0 events per 1000 person years; hazard ratio 0.67, 0.32 to 1.41). Secondary outcomes generated similar results but with wider confidence intervals.
Conclusions: In this cohort study, the GLP-1 receptor agonist-SGLT-2 inhibitor combination was associated with a lower risk of major adverse cardiovascular events and serious renal events compared with either drug class alone.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: this study was funded by the Canadian Institutes of Health Research; LA received speaking and consulting fees from Pfizer and Roche for work unrelated to this project; SS attended scientific advisory committee meetings for AstraZeneca, Atara, Bristol-Myers-Squibb, Merck, Novartis, Panalgo, Pfizer, and Seqirus and received speaking fees from Boehringer-Ingelheim and Novartis; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
