Predicting response to non-selective beta-blockers with liver-spleen stiffness and heart rate in patients with liver cirrhosis and high-risk varices
- PMID: 38664292
- PMCID: PMC12003444
- DOI: 10.1007/s12072-024-10649-7
Predicting response to non-selective beta-blockers with liver-spleen stiffness and heart rate in patients with liver cirrhosis and high-risk varices
Abstract
Introduction: Non-selective beta-blockers (NSBB) are used for primary prophylaxis in patients with liver cirrhosis and high-risk varices (HRVs). Assessing therapeutic response is challenging due to the invasive nature of hepatic venous pressure gradient (HVPG) measurement. This study aims to define a noninvasive machine-learning based approach to determine response to NSBB in patients with liver cirrhosis and HRVs.
Methods: We conducted a prospective study on a cohort of cirrhotic patients with documented HRVs receiving NSBB treatment. Patients were followed-up with clinical and elastography appointments at 3, 6, and 12 months after NSBB treatment initiation. NSBB response was defined as stationary or downstaging variceal grading at the 12-month esophagogastroduodenoscopy (EGD). In contrast, non-response was defined as upstaging variceal grading at the 12-month EGD or at least one variceal hemorrhage episode during the 12-month follow-up. We chose cut-off values for univariate and multivariate model with 100% specificity.
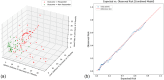
Results: According to least absolute shrinkage and selection operator (LASSO) regression, spleen stiffness (SS) and liver stiffness (LS) percentual decrease, along with changes in heart rate (HR) at 3 months were the most significant predictors of NSBB response. A decrease > 11.5% in SS, > 16.8% in LS, and > 25.3% in HR was associated with better prediction of clinical response to NSBB. SS percentual decrease showed the highest accuracy (86.4%) with high sensitivity (78.8%) when compared to LS and HR. The multivariate model incorporating SS, LS, and HR showed the highest discrimination and calibration metrics (AUROC = 0.96), with the optimal cut-off of 0.90 (sensitivity 94.2%, specificity 100%, PPV 95.7%, NPV 100%, accuracy 97.5%).
Keywords: Elastography; Hepatic venous pressure gradient (HVPG); High-risk varices; Liver cirrhosis; Liver stiffness (LS); Machine learning; Non-selective beta-blockers (NSBB); Primary prophylaxis; Spleen stiffness (SS); Variceal hemorrhage.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Mauro Giuffrè, Johannes Dupont, Alessia Visintin, Flora Masutti, Fabio Monica, Kisung You, Dennis L. Shung, Lory Saveria Crocè have no conflict of interest to declare. Ethical approval: The study was conducted in accordance with the ethical principles for medical research involving human subjects as indicated by the Declaration of Helsinki. The study was carried out following the guidelines of the local Ethics Committee for conducting research involving humans (Protocol Number: 2783). Informed consent: Informed consent was obtained for each patient participating in the study.
Figures

Comment in
-
Expanding the utility of non-invasive NSBB monitoring in early CSPH and beyond.Hepatol Int. 2025 Jun 26. doi: 10.1007/s12072-025-10862-y. Online ahead of print. Hepatol Int. 2025. PMID: 40569565 No abstract available.
References
-
- Garcia-Tsao G, Abraldes JG, Berzigotti A. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. - PubMed
-
- Ferral H, Garcia-Pagàn JC, Schepis F. HVPG as a gold standard: consensus statements of panel 1. Portal hypertension VII. Springer International Publishing: Cham; 2022. p. 61–4 .10.1007/978-3-031-08552-9_6
-
- Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399–411. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

