Cross-species spill-over potential of the H9N2 bat influenza A virus
- PMID: 38664384
- PMCID: PMC11045754
- DOI: 10.1038/s41467-024-47635-4
Cross-species spill-over potential of the H9N2 bat influenza A virus
Abstract
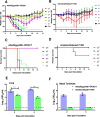
In 2017, a novel influenza A virus (IAV) was isolated from an Egyptian fruit bat. In contrast to other bat influenza viruses, the virus was related to avian A(H9N2) viruses and was probably the result of a bird-to-bat transmission event. To determine the cross-species spill-over potential, we biologically characterize features of A/bat/Egypt/381OP/2017(H9N2). The virus has a pH inactivation profile and neuraminidase activity similar to those of human-adapted IAVs. Despite the virus having an avian virus-like preference for α2,3 sialic acid receptors, it is unable to replicate in male mallard ducks; however, it readily infects ex-vivo human respiratory cell cultures and replicates in the lungs of female mice. A/bat/Egypt/381OP/2017 replicates in the upper respiratory tract of experimentally-infected male ferrets featuring direct-contact and airborne transmission. These data suggest that the bat A(H9N2) virus has features associated with increased risk to humans without a shift to a preference for α2,6 sialic acid receptors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Li, J., Lai, S., Gao, G. F. & Shi, W. The emergence, genomic diversity and global spread of SARS-CoV−2. Nature600, 408–418 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

