Ultrastructural localization of Porphyromonas gingivalis gingipains in the substantia nigra of Parkinson's disease brains
- PMID: 38664405
- PMCID: PMC11045759
- DOI: 10.1038/s41531-024-00705-2
Ultrastructural localization of Porphyromonas gingivalis gingipains in the substantia nigra of Parkinson's disease brains
Abstract
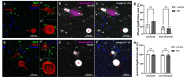
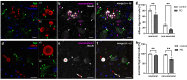
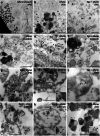
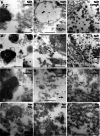
Gingipains are protease virulence factors produced by Porphyromonas gingivalis, a Gram-negative bacterium best known for its role in chronic periodontitis. Gingipains were recently identified in the middle temporal gyrus of postmortem Alzheimer's disease (AD) brains, where gingipain load correlated with AD diagnosis and tau and ubiquitin pathology. Since AD and Parkinson's disease (PD) share some overlapping pathologic features, including nigral pathology and Lewy bodies, the current study explored whether gingipains are present in the substantia nigra pars compacta of PD brains. In immunohistochemical techniques and multi-channel fluorescence studies, gingipain antigens were abundant in dopaminergic neurons in the substantia nigra of both PD and neurologically normal control brains. 3-dimensional reconstructions of Lewy body containing neurons revealed that gingipains associated with the periphery of alpha-synuclein aggregates but were occasionally observed inside aggregates. In vitro proteomic analysis demonstrated that recombinant alpha-synuclein is cleaved by lysine-gingipain, generating multiple alpha-synuclein fragments including the non-amyloid component fragments. Immunogold electron microscopy with co-labeling of gingipains and alpha-synuclein confirmed the occasional colocalization of gingipains with phosphorylated (pSER129) alpha-synuclein. In dopaminergic neurons, gingipains localized to the perinuclear cytoplasm, neuromelanin, mitochondria, and nucleus. These data suggest that gingipains localize in dopaminergic neurons in the substantia nigra and interact with alpha-synuclein.
© 2024. The Author(s).
Conflict of interest statement
F.E. and S.S.D. were employees of Cortexyme at the time the research was conducted. Dominy is an inventor on gingipain inhibitor patents, and cofounder of Lighthouse Pharmaceuticals, a company developing gingipain inhibitors as therapeutics. The other authors declare that they have no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

