Absence of calcium-sensing receptor basal activity due to inter-subunit disulfide bridges
- PMID: 38664468
- PMCID: PMC11045811
- DOI: 10.1038/s42003-024-06189-3
Absence of calcium-sensing receptor basal activity due to inter-subunit disulfide bridges
Abstract
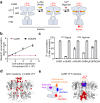
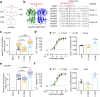
G protein-coupled receptors naturally oscillate between inactive and active states, often resulting in receptor constitutive activity with important physiological consequences. Among the class C G protein-coupled receptors that typically sense amino-acids and their derivatives, the calcium sensing receptor (CaSR) tightly controls blood calcium levels. Its constitutive activity has not yet been studied. Here, we demonstrate the importance of the inter-subunit disulfide bridges in maintaining the inactive state of CaSR, resulting in undetectable constitutive activity, unlike the other class C receptors. Deletion of these disulfide bridges results in strong constitutive activity that is abolished by mutations preventing amino acid binding. It shows that this inter-subunit disulfide link is necessary to limit the agonist effect of amino acids on CaSR. Furthermore, human genetic mutations deleting these bridges and associated with hypocalcemia result in elevated CaSR constitutive activity. These results highlight the physiological importance of fine tuning the constitutive activity of G protein-coupled receptors.
© 2024. The Author(s).
Conflict of interest statement
Philippe Rondard and Jean-Philippe Pin are involved in a collaborative team between the CNRS and Revvity (IGF, Montpellier). All other authors declare no competing interests.
Figures





Similar articles
-
Calcium-Sensing Receptor Internalization Is β-Arrestin-Dependent and Modulated by Allosteric Ligands.Mol Pharmacol. 2019 Oct;96(4):463-474. doi: 10.1124/mol.119.116772. Epub 2019 Aug 9. Mol Pharmacol. 2019. PMID: 31399503
-
Two novel mutations of the calcium-sensing receptor gene affecting the same amino acid position lead to opposite phenotypes and reveal the importance of p.N802 on receptor activity.Eur J Endocrinol. 2013 Jan 17;168(2):K27-34. doi: 10.1530/EJE-12-0714. Print 2013 Feb. Eur J Endocrinol. 2013. PMID: 23169696
-
Monitoring Calcium-Sensing Receptor (CaSR)-Induced Intracellular Calcium Flux Using an Indo-1 Flow Cytometry Assay.Methods Mol Biol. 2025;2861:43-55. doi: 10.1007/978-1-0716-4164-4_4. Methods Mol Biol. 2025. PMID: 39395096
-
Insights into calcium-sensing receptor trafficking and biased signalling by studies of calcium homeostasis.J Mol Endocrinol. 2018 Jul;61(1):R1-R12. doi: 10.1530/JME-18-0049. Epub 2018 Mar 29. J Mol Endocrinol. 2018. PMID: 29599414 Review.
-
Activating Calcium-Sensing Receptor Mutations: Prospects for Future Treatment with Calcilytics.Trends Endocrinol Metab. 2016 Sep;27(9):643-652. doi: 10.1016/j.tem.2016.05.005. Epub 2016 Jun 20. Trends Endocrinol Metab. 2016. PMID: 27339034 Review.
Cited by
-
The calcium-sensing receptor: a comprehensive review on its role in calcium homeostasis and therapeutic implications.Am J Transl Res. 2025 Mar 15;17(3):2322-2338. doi: 10.62347/QGTS5711. eCollection 2025. Am J Transl Res. 2025. PMID: 40226019 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- EQU202303016470/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
- 31070737/National Natural Science Foundation of China (National Science Foundation of China)
- 31371423/National Natural Science Foundation of China (National Science Foundation of China)
- 32330049/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

