ISWI catalyzes nucleosome sliding in condensed nucleosome arrays
- PMID: 38664566
- PMCID: PMC7618249
- DOI: 10.1038/s41594-024-01290-x
ISWI catalyzes nucleosome sliding in condensed nucleosome arrays
Abstract
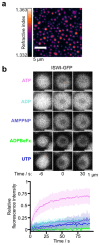
How chromatin enzymes work in condensed chromatin and how they maintain diffusional mobility inside remains unexplored. Here we investigated these challenges using the Drosophila ISWI remodeling ATPase, which slides nucleosomes along DNA. Folding of chromatin fibers did not affect sliding in vitro. Catalytic rates were also comparable in- and outside of chromatin condensates. ISWI cross-links and thereby stiffens condensates, except when ATP hydrolysis is possible. Active hydrolysis is also required for ISWI's mobility in condensates. Energy from ATP hydrolysis therefore fuels ISWI's diffusion through chromatin and prevents ISWI from cross-linking chromatin. Molecular dynamics simulations of a 'monkey-bar' model in which ISWI grabs onto neighboring nucleosomes, then withdraws from one before rebinding another in an ATP hydrolysis-dependent manner, qualitatively agree with our data. We speculate that monkey-bar mechanisms could be shared with other chromatin factors and that changes in chromatin dynamics caused by mutations in remodelers could contribute to pathologies.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
We declare no competing interest.
Figures








Update of
-
ISWI catalyzes nucleosome sliding in condensed nucleosome arrays.bioRxiv [Preprint]. 2023 Dec 4:2023.12.04.569516. doi: 10.1101/2023.12.04.569516. bioRxiv. 2023. Update in: Nat Struct Mol Biol. 2024 Sep;31(9):1331-1340. doi: 10.1038/s41594-024-01290-x. PMID: 38106060 Free PMC article. Updated. Preprint.
References
-
- Post CB, Zimm BH. Theory of DNA condensation: Collapse versus aggregation. Biopolymers. 1982;21:2123–2137. - PubMed
-
- Woodcock CLF. Ultrastructure of inactive chromatin. Journal of Cell Biology. 1973;59:A368
-
- Olins AL, Olins DE. Spheroid chromatin units (v bodies) Science (1979) 1974;183:330–332. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

