Spatiotemporal patterns of locus coeruleus integrity predict cortical tau and cognition
- PMID: 38664576
- PMCID: PMC11108787
- DOI: 10.1038/s43587-024-00626-y
Spatiotemporal patterns of locus coeruleus integrity predict cortical tau and cognition
Abstract
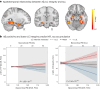
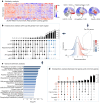
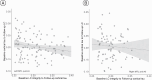
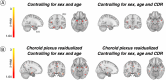
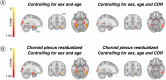
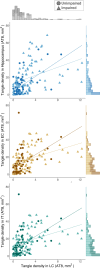
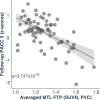
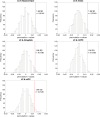
Autopsy studies indicated that the locus coeruleus (LC) accumulates hyperphosphorylated tau before allocortical regions in Alzheimer's disease. By combining in vivo longitudinal magnetic resonance imaging measures of LC integrity, tau positron emission tomography imaging and cognition with autopsy data and transcriptomic information, we examined whether LC changes precede allocortical tau deposition and whether specific genetic features underlie LC's selective vulnerability to tau. We found that LC integrity changes preceded medial temporal lobe tau accumulation, and together these processes were associated with lower cognitive performance. Common gene expression profiles between LC-medial temporal lobe-limbic regions map to biological functions in protein transport regulation. These findings advance our understanding of the spatiotemporal patterns of initial tau spreading from the LC and LC's selective vulnerability to Alzheimer's disease pathology. LC integrity measures can be a promising indicator for identifying the time window when individuals are at risk of disease progression and underscore the importance of interventions mitigating initial tau spread.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- P30AG10161/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P41 EB022544/EB/NIBIB NIH HHS/United States
- R01AG068062/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P01 AG036694/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- R01AG061445/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AG062559/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- S10 OD010364/OD/NIH HHS/United States
- R01AG15819/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AG068062/AG/NIA NIH HHS/United States
- R01AG1791/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AG046396/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AG046396/AG/NIA NIH HHS/United States
- R21AG074220/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- S10 RR021110/RR/NCRR NIH HHS/United States
- R01 AG061811/AG/NIA NIH HHS/United States
- P01AG036694/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R21 AG074220/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- R01AG061811/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AG061445/AG/NIA NIH HHS/United States
- R01AG082006/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AG082006/AG/NIA NIH HHS/United States
- R01 AG062559/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

