Minimizing higher-order aggregation maximizes iron mobilization by small molecules
- PMID: 38664586
- PMCID: PMC11831690
- DOI: 10.1038/s41589-024-01596-3
Minimizing higher-order aggregation maximizes iron mobilization by small molecules
Abstract
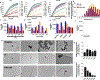
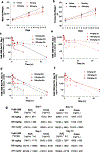
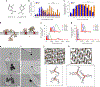
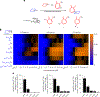
The natural product hinokitiol mobilizes iron across lipid bilayers at low concentrations and restores hemoglobinization in iron transporter protein-deficient systems. But hinokitiol fails to similarly mobilize iron at higher concentrations, limiting its uses in chemical biology and medicine. Here we show that at higher concentrations, hinokitiol3:Fe(III) complexes form large, higher-order aggregates, leading to loss of transmembrane iron mobilization. Guided by this understanding and systematic structure-function studies enabled by modular synthesis, we identified FeM-1269, which minimally aggregates and dose-dependently mobilizes iron across lipid bilayers even at very high concentrations. In contrast to hinokitiol, FeM-1269 is also well-tolerated in animals at high doses for extended periods of time. In a mouse model of anemia of inflammation, FeM-1269 increases serum iron, transferrin saturation, hemoglobin and hematocrit. This rationally developed iron-mobilizing small molecule has enhanced potential as a molecular prosthetic for understanding and potentially treating iron transporter deficiencies.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
M.D.B. is an inventor on the US patent application pub. 20180263926, ‘Restoring Physiology in Iron-Deficient Organisms Using Small Molecules’, which is directed to the use of small-molecule iron transporters to treat deficiencies of iron-transport proteins. A.D.B., A.M.S.M. and M.D.B. are inventors on the US patent application pub. 20210163393, ‘Hinokitiol analogs, methods of preparing and pharmaceutical compositions thereof’, which is directed to analogs of hinokitiol, preparation and formulation. These patent applications, their foreign counterparts and others have been licensed by Cajal Neuroscience, for which M.D.B. is a scientific advisor and shareholder. Ambys Medicines has filed patent applications directed to tropolone derivatives and tautomers thereof for iron regulation in animals. J.C., N.M.S., D.M.M., T.G., A.O. and S.J.H. were full-time employees of Ambys Medicines when these data were generated. These patents are currently owned by Cajal Neuroscience. The other authors declare no competing interests.
Figures
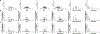









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

