Integrated metabolome and transcriptome analyses reveal the role of BoGSTF12 in anthocyanin accumulation in Chinese kale (Brassica oleracea var. alboglabra)
- PMID: 38664614
- PMCID: PMC11044404
- DOI: 10.1186/s12870-024-05016-5
Integrated metabolome and transcriptome analyses reveal the role of BoGSTF12 in anthocyanin accumulation in Chinese kale (Brassica oleracea var. alboglabra)
Abstract
Background: The vivid red, purple, and blue hues that are observed in a variety of plant fruits, flowers, and leaves are produced by anthocyanins, which are naturally occurring pigments produced by a series of biochemical processes occurring inside the plant cells. The purple-stalked Chinese kale, a popular vegetable that contains anthocyanins, has many health benefits but needs to be investigated further to identify the genes involved in the anthocyanin biosynthesis and translocation in this vegetable.
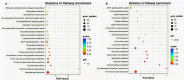
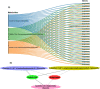
Results: In this study, the purple- and green-stalked Chinese kale were examined using integrative transcriptome and metabolome analyses. The content of anthocyanins such as cyanidin-3-O-(6″-O-feruloyl) sophoroside-5-O-glucoside, cyanidin-3,5-O-diglucoside (cyanin), and cyanidin-3-O-(6″-O-p-hydroxybenzoyl) sophoroside-5-O-glucoside were considerably higher in purple-stalked Chinese kale than in its green-stalked relative. RNA-seq analysis indicated that 23 important anthocyanin biosynthesis genes, including 3 PAL, 2 C4H, 3 4CL, 3 CHS, 1 CHI, 1 F3H, 2 FLS, 2 F3'H, 1 DFR, 3 ANS, and 2 UFGT, along with the transcription factor BoMYB114, were significantly differentially expressed between the purple- and green-stalked varieties. Results of analyzing the expression levels of 11 genes involved in anthocyanin production using qRT-PCR further supported our findings. Association analysis between genes and metabolites revealed a strong correlation between BoGSTF12 and anthocyanin. We overexpressed BoGSTF12 in Arabidopsis thaliana tt19, an anthocyanin transport mutant, and this rescued the anthocyanin-loss phenotype in the stem and rosette leaves, indicating BoGSTF12 encodes an anthocyanin transporter that affects the accumulation of anthocyanins.
Conclusion: This work represents a key step forward in our understanding of the molecular processes underlying anthocyanin production in Chinese kale. Our comprehensive metabolomic and transcriptome analyses provide important insights into the regulatory system that controls anthocyanin production and transport, while providing a foundation for further research to elucidate the physiological importance of the metabolites found in this nutritionally significant vegetable.
Keywords: Brassica oleracea; Anthocyanins; Metabolome; RNA-seq; qRT-PCR.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Aires A. Brassica composition and food processing. Processing and Impact on active components in Food. Elsevier; 2015. pp. 17–25.
-
- Jahangir M, Kim HK, Choi YH, Verpoorte R. Health-affecting compounds in Brassicaceae. CRFSFS. 2009;8(2):31–43.
-
- Paiva SA, Russell RM. β-carotene and other carotenoids as antioxidants. JACN. 1999;18(5):426–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

