Oral mouthwashes for asymptomatic to mildly symptomatic adults with COVID-19 and salivary viral load: a randomized, placebo-controlled, open-label clinical trial
- PMID: 38664718
- PMCID: PMC11044332
- DOI: 10.1186/s12903-024-04246-1
Oral mouthwashes for asymptomatic to mildly symptomatic adults with COVID-19 and salivary viral load: a randomized, placebo-controlled, open-label clinical trial
Abstract
Background: Recent randomized clinical trials suggest that the effect of using cetylpyridinium chloride (CPC) mouthwashes on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in COVID-19 patients has been inconsistent. Additionally, no clinical study has investigated the effectiveness of on-demand aqueous chlorine dioxide mouthwash against COVID-19.
Methods: We performed a randomized, placebo-controlled, open-label clinical trial to assess for any effects of using mouthwash on the salivary SARS-CoV-2 viral load among asymptomatic to mildly symptomatic adult COVID-19-positive patients. Patients were randomized to receive either 20 mL of 0.05% CPC, 10 mL of 0.01% on-demand aqueous chlorine dioxide, or 20 mL of placebo mouthwash (purified water) in a 1:1:1 ratio. The primary endpoint was the cycle threshold (Ct) values employed for SARS-CoV-2 salivary viral load estimation. We used linear mixed-effects models to assess for any effect of the mouthwashes on SARS-CoV-2 salivary viral load.
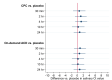
Results: Of a total of 96 eligible participants enrolled from November 7, 2022, to January 19, 2023, 90 were accepted for the primary analysis. The use of 0.05% CPC mouthwash was not shown to be superior to placebo in change from baseline salivary Ct value at 30 min (difference vs. placebo, 0.640; 95% confidence interval [CI], -1.425 to 2.706; P = 0.543); 2 h (difference vs. placebo, 1.158; 95% CI, -0.797 to 3.112; P = 0.246); 4 h (difference vs. placebo, 1.283; 95% CI, -0.719 to 3.285; P = 0.209); 10 h (difference vs. placebo, 0.304; 95% CI, -1.777 to 2.385; P = 0.775); or 24 h (difference vs. placebo, 0.782; 95% CI, -1.195 to 2.759; P = 0.438). The use of 0.01% on-demand aqueous chlorine dioxide mouthwash was also not shown to be superior to placebo in change from baseline salivary Ct value at 30 min (difference vs. placebo, 0.905; 95% CI, -1.079 to 2.888; P = 0.371); 2 h (difference vs. placebo, 0.709; 95% CI, -1.275 to 2.693; P = 0.483); 4 h (difference vs. placebo, 0.220; 95% CI, -1.787 to 2.226; P = 0.830); 10 h (difference vs. placebo, 0.198; 95% CI, -1.901 to 2.296; P = 0.854); or 24 h (difference vs. placebo, 0.784; 95% CI, -1.236 to 2.804; P = 0.447).
Conclusions: In asymptomatic to mildly symptomatic adults with COVID-19, compared to placebo, the use of 0.05% CPC and 0.01% on-demand aqueous chlorine dioxide mouthwash did not lead to a significant reduction in SARS-CoV-2 salivary viral load. Future studies of the efficacy of CPC and on-demand aqueous chlorine dioxide mouthwash on the viral viability of SARS-CoV-2 should be conducted using different specimen types and in multiple populations and settings.
Keywords: COVID-19; Cetylpyridinium chloride; Mouthwash; On-demand aqueous chlorine dioxide solution; Randomized clinical trial.
© 2024. The Author(s).
Conflict of interest statement
S.K., D.O., S.T., and K.K. have received research support from Earth Corporation, Tokyo, Japan. H.M., H.Y., S.H., S.Y., R.M.S., and K.S. declare no competing interests.
Figures
Similar articles
-
A MULTICENTER, RANDOMIZED, OPEN-LABEL, PLACEBO-CONTROLLED CLINICAL TRIAL OF THE EFFECT OF CETYLPYRIDINIUM CHLORIDE (CPC) MOUTHWASH AND ON-DEMAND AQUEOUS CHLORINE DIOXIDE MOUTHWASH ON SARS-COV-2 VIRAL TITER IN PATIENTS WITH MILD COVID-19.J Evid Based Dent Pract. 2024 Dec;24(4):102040. doi: 10.1016/j.jebdp.2024.102040. Epub 2024 Sep 17. J Evid Based Dent Pract. 2024. PMID: 39631972 Clinical Trial.
-
Protocol for a Randomized, Open-Label Clinical Trial on the Effect of Mouthwash on Salivary SARS-CoV-2 Load.Life (Basel). 2023 Dec 8;13(12):2312. doi: 10.3390/life13122312. Life (Basel). 2023. PMID: 38137913 Free PMC article.
-
Efficacy of Cetylpyridinium Chloride mouthwash against SARS-CoV-2: A systematic review of randomized controlled trials.Mol Oral Microbiol. 2023 Jun;38(3):171-180. doi: 10.1111/omi.12408. Epub 2023 Mar 3. Mol Oral Microbiol. 2023. PMID: 36808889
-
Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: A Double-Blind Randomized Clinical Trial.J Dent Res. 2022 Nov;101(12):1450-1456. doi: 10.1177/00220345221102310. Epub 2022 Jun 21. J Dent Res. 2022. PMID: 35727681 Clinical Trial.
-
Efficacy of mouthwash on reducing salivary SARS-CoV-2 viral load and clinical symptoms: a systematic review and meta-analysis.BMC Infect Dis. 2023 Oct 11;23(1):678. doi: 10.1186/s12879-023-08669-z. BMC Infect Dis. 2023. PMID: 37821800 Free PMC article.
Cited by
-
Antiseptics as effective virucidal agents against SARS-CoV-2: Systematic review and Bayesian network meta-analysis.Jpn Dent Sci Rev. 2025 Dec;61:138-154. doi: 10.1016/j.jdsr.2025.05.001. Epub 2025 Jun 10. Jpn Dent Sci Rev. 2025. PMID: 40547479 Free PMC article. Review.
References
-
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. 2023. Available: https://covid19.who.int/. (Accessed 2023 6 December).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous