The biophysical function of pulmonary surfactant
- PMID: 38664968
- PMCID: PMC11213971
- DOI: 10.1016/j.bpj.2024.04.021
The biophysical function of pulmonary surfactant
Abstract
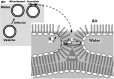
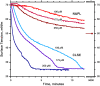
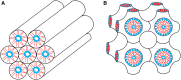
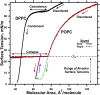
The type II pneumocytes of the lungs secrete a mixture of lipids and proteins that together acts as a surfactant. The material forms a thin film on the surface of the liquid layer that lines the alveolar air sacks. When compressed by the decreasing alveolar surface area during exhalation, the films reduce surface tension to exceptionally low levels. Pulmonary surfactant is essential for preserving the integrity of the barrier between alveolar air and capillary blood during normal breathing. This review focuses on the major biophysical processes by which endogenous pulmonary surfactant achieves its function and the mechanisms involved in those processes. Vesicles of pulmonary surfactant adsorb rapidly from the alveolar liquid to form the interfacial film. Interfacial insertion, which requires the hydrophobic surfactant protein SP-B, proceeds by a process analogous to the fusion of two vesicles. When compressed, the adsorbed film desorbs slowly. Constituents remain at the surface at high interfacial concentrations that reduce surface tensions well below equilibrium levels. We review the models proposed to explain how pulmonary surfactant achieves both the rapid adsorption and slow desorption characteristic of a functional film.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Bastacky J., Lee C.Y., et al. Clements J.A. Alveolar lining layer is thin and continuous: low-temperature scanning electron microscopy of rat lung. J. Appl. Physiol. 1995;79:1615–1628. - PubMed
-
- Wright J.R. Immunomodulatory functions of surfactant. Physiol. Rev. 1997;77:931–962. - PubMed
-
- Clements J.A. Functions of the alveolar lining. Am. Rev. Respir. Dis. 1977;115(6 part 2):67–71. - PubMed
-
- Lachmann B., Grossmann G., et al. Robertson B. Lung mechanics during spontaneous ventilation in premature and fullterm rabbit neonates. Respir. Physiol. 1979;38:283–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

