Gauging Iron-Sulfur Cubane Reactivity from Covalency: Trends with Oxidation State
- PMID: 38665672
- PMCID: PMC11040707
- DOI: 10.1021/jacsau.4c00213
Gauging Iron-Sulfur Cubane Reactivity from Covalency: Trends with Oxidation State
Abstract
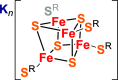
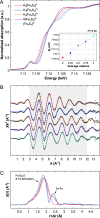
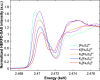
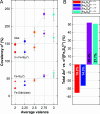
We investigated room-temperature metal and ligand K-edge X-ray absorption (XAS) spectra of a complete redox series of cubane-type iron-sulfur clusters. The Fe K-edge position provides a qualitative but convenient alternative to the traditional spectroscopic descriptors used to identify oxidation states in these systems, which we demonstrate by providing a calibration curve based on two analytic methods. Furthermore, high energy resolution fluorescence detected XAS (HERFD-XAS) at the S K-edge was used to measure Fe-S bond covalencies and record their variation with the average valence of the Fe atoms. While the Fe-S(thiolate) covalency evolves linearly, gaining 11 ± 0.4% per bond and hole, the Fe-S(μ3) covalency evolves asystematically, reflecting changes in the magnetic exchange mechanism. A strong discontinuity manifested for superoxidation to the all-ferric state, distinguishing its electronic structure and its potential (bio)chemical role from those of its redox congeners. We highlight the functional implications of these trends for the reactivity of iron-sulfur cubanes.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Kubas A. Characterization of charge transfer excited states in [2Fe–2S] iron–sulfur clusters using conventional configuration interaction techniques. Theor. Chem. Acc. 2020, 139 (7), 120.10.1007/s00214-020-02635-7. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
