Evaluating the Efficacy and Safety of Tirzepatide on Glycaemic and Non-glycaemic Outcomes in Diabetes: A Systematic Review of Meta-Analyses
- PMID: 38665722
- PMCID: PMC11044191
- DOI: 10.7759/cureus.56939
Evaluating the Efficacy and Safety of Tirzepatide on Glycaemic and Non-glycaemic Outcomes in Diabetes: A Systematic Review of Meta-Analyses
Abstract
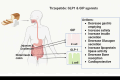
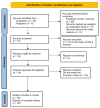
Tirzepatide is a novel once-a-week dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, recently approved for type 2 diabetes mellitus (T2DM) and obesity. A systematic review of the literature published in multiple meta-analyses on Tirzepatide with emphasis on its effect on glycaemic and non-glycaemic parameters was conducted. We systematically searched the electronic databases PubMed and Google Scholar up to August 2023 for meta-analyses that compared Tirzepatide with placebo or active antihyperglycaemic drugs in subjects with T2DM. Various parameters for efficacy and safety, with their point estimates and confidence intervals, such as glycated haemoglobin (HbA1c), fasting serum glucose (FSG), body weight, lipid, and cardiovascular outcomes were assessed. Six meta-analyses fulfilled the pre-specified criteria and were included in the study. In all the studies, Tirzepatide treatment at different doses resulted in a significant reduction in HbA1c and FSG levels along with a significant reduction in weight compared with active control and placebo groups. Tirzepatide significantly reduced levels of triglycerides and increased high-density lipoprotein (HDL) cholesterol, whether used as monotherapy or add-on therapy. The studies suggested the cardiovascular safety of Tirzepatide as there was no increase in major adverse cardiovascular events (MACE). The drug shows lesser hypoglycemia but predominant gastrointestinal adverse effects such as nausea, vomiting, and diarrhoea. In conclusion, Tirzepatide shows superior glycaemic control and weight loss in patients with T2DM with beneficial effects on lipids, without an increased risk of hypoglycemia and cardiovascular events.
Keywords: body weight; dual agonist; glycaemic control; incretins; tirzepatide; type 2 diabetes mellitus.
Copyright © 2024, Kaore et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Diabetes. [ Jun; 2023 ]. https://www.who.int/health-topics/diabetes https://www.who.int/health-topics/diabetes
-
- Incretin hormones: their role in health and disease. Nauck MA, Meier JJ. Diabetes Obes Metab. 2018;20 Suppl 1:5–21. - PubMed
-
- Biology of incretins: GLP-1 and GIP. Baggio LL, Drucker DJ. Gastroenterology. 2007;132:2131–2157. - PubMed
-
- Glucagon-like peptide 1 in health and disease. Andersen A, Lund A, Knop FK, Vilsbøll T. Nat Rev Endocrinol. 2018;14:390–403. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
