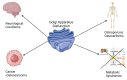
Exploring the Implications of Golgi Apparatus Dysfunction in Bone Diseases
- PMID: 38665758
- PMCID: PMC11045246
- DOI: 10.7759/cureus.56982
Exploring the Implications of Golgi Apparatus Dysfunction in Bone Diseases
Abstract
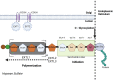
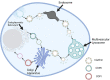
The Golgi apparatus is an organelle responsible for protein processing, sorting, and transport in cells. Recent research has shed light on its possible role in the pathogenesis of various bone diseases. This review seeks to explore its significance in osteoporosis, osteogenesis imperfecta, and other bone conditions such as dysplasias. Numerous lines of evidence demonstrate that perturbations to Golgi apparatus function can disrupt post-translational protein modification, folding and trafficking functions crucial for bone formation, mineralization, and remodeling. Abnormalities related to glycosylation, protein sorting, or vesicular transport in Golgi have been associated with altered osteoblast and osteoclast function, compromised extracellular matrix composition, as well as disrupted signaling pathways involved with homeostasis of bones. Mutations or dysregulation of Golgi-associated proteins, including golgins and coat protein complex I and coat protein complex II coat components, have also been implicated in bone diseases. Such genetic alterations may disrupt Golgi structure, membrane dynamics, and protein transport, leading to bone phenotype abnormalities. Understanding the links between Golgi apparatus dysfunction and bone diseases could provide novel insights into disease pathogenesis and potential therapeutic targets. Future research should focus on unraveling specific molecular mechanisms underlying Golgi dysfunction associated with bone diseases to develop targeted interventions for restoring normal bone homeostasis while decreasing clinical manifestations associated with these issues.
Keywords: bone diseases; glycosylation; golgi apparatus; protein sorting; signaling pathways; vesicular trafficking.
Copyright © 2024, Iacobescu et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Camillo Golgi and the discovery of the Golgi apparatus. Dröscher A. Histochem Cell Biol. 1998;109:425–430. - PubMed
-
- Unraveling molecular and genetic insights into neurodegenerative diseases: advances in understanding Alzheimer's, Parkinson's, and Huntington's diseases and amyotrophic lateral sclerosis. Ciurea AV, Mohan AG, Covache-Busuioc RA, Costin HP, Glavan LA, Corlatescu AD, Saceleanu VM. Int J Mol Sci. 2023;24:10809. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
