Aβ oligomers peak in early stages of Alzheimer's disease preceding tau pathology
- PMID: 38666085
- PMCID: PMC11044868
- DOI: 10.1002/dad2.12589
Aβ oligomers peak in early stages of Alzheimer's disease preceding tau pathology
Erratum in
-
Correction to "Aβ oligomers peak in early stages of Alzheimer's disease preceding tau pathology".Alzheimers Dement (Amst). 2024 May 21;16(2):e12599. doi: 10.1002/dad2.12599. eCollection 2024 Apr-Jun. Alzheimers Dement (Amst). 2024. PMID: 38774606 Free PMC article.
Abstract
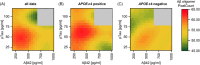
Introduction: Soluble amyloid beta (Aβ) oligomers have been suggested as initiating Aβ related neuropathologic change in Alzheimer's disease (AD) but their quantitative distribution and chronological sequence within the AD continuum remain unclear.
Methods: A total of 526 participants in early clinical stages of AD and controls from a longitudinal cohort were neurobiologically classified for amyloid and tau pathology applying the AT(N) system. Aβ and tau oligomers in the quantified cerebrospinal fluid (CSF) were measured using surface-based fluorescence intensity distribution analysis (sFIDA) technology.
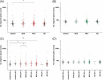
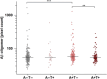
Results: Across groups, highest Aβ oligomer levels were found in A+ with subjective cognitive decline and mild cognitive impairment. Aβ oligomers were significantly higher in A+T- compared to A-T- and A+T+. APOE ε4 allele carriers showed significantly higher Aβ oligomer levels. No differences in tau oligomers were detected.
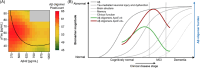
Discussion: The accumulation of Aβ oligomers in the CSF peaks early within the AD continuum, preceding tau pathology. Disease-modifying treatments targeting Aβ oligomers might have the highest therapeutic effect in these disease stages.
Highlights: Using surface-based fluorescence intensity distribution analysis (sFIDA) technology, we quantified Aβ oligomers in cerebrospinal fluid (CSF) samples of the DZNE-Longitudinal Cognitive Impairment and Dementia (DELCODE) cohortAβ oligomers were significantly elevated in mild cognitive impairment (MCI)Amyloid-positive subjects in the subjective cognitive decline (SCD) group increased compared to the amyloid-negative control groupInterestingly, levels of Aβ oligomers decrease at advanced stages of the disease (A+T+), which might be explained by altered clearing mechanisms.
Keywords: APOE; AT(N) classification; Alzheimer's disease; Aβ; cerebrospinal fluid; oligomers; preclinical; prodromal; sFIDA; tau.
© 2024 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dieter Willbold and Oliver Bannach are co‐founders and shareholders of attyloid GmbH. This had no influence of the interpretation of the data. All other authors declare no competing interests related to this work. The sFIDA method is protected by patents EP3271724A1, EP3014279B1 and EP2794655B1. Author disclosures are available in the supporting information.
Figures
References
-
- Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357‐367. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
