The right inferior frontal gyrus as pivotal node and effective regulator of the basal ganglia-thalamocortical response inhibition circuit
- PMID: 38666118
- PMCID: PMC10917375
- DOI: 10.1093/psyrad/kkad016
The right inferior frontal gyrus as pivotal node and effective regulator of the basal ganglia-thalamocortical response inhibition circuit
Abstract
Background: The involvement of specific basal ganglia-thalamocortical circuits in response inhibition has been extensively mapped in animal models. However, the pivotal nodes and directed causal regulation within this inhibitory circuit in humans remains controversial.
Objective: The main aim of the present study was to determine the causal information flow and critical nodes in the basal ganglia-thalamocortical inhibitory circuits and also to examine whether these are modulated by biological factors (i.e. sex) and behavioral performance.
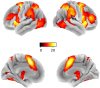
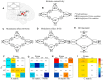
Methods: Here, we capitalize on the recent progress in robust and biologically plausible directed causal modeling (DCM-PEB) and a large response inhibition dataset (n = 250) acquired with concomitant functional magnetic resonance imaging to determine key nodes, their causal regulation and modulation via biological variables (sex) and inhibitory performance in the inhibitory circuit encompassing the right inferior frontal gyrus (rIFG), caudate nucleus (rCau), globus pallidum (rGP), and thalamus (rThal).
Results: The entire neural circuit exhibited high intrinsic connectivity and response inhibition critically increased causal projections from the rIFG to both rCau and rThal. Direct comparison further demonstrated that response inhibition induced an increasing rIFG inflow and increased the causal regulation of this region over the rCau and rThal. In addition, sex and performance influenced the functional architecture of the regulatory circuits such that women displayed increased rThal self-inhibition and decreased rThal to GP modulation, while better inhibitory performance was associated with stronger rThal to rIFG communication. Furthermore, control analyses did not reveal a similar key communication in a left lateralized model.
Conclusions: Together, these findings indicate a pivotal role of the rIFG as input and causal regulator of subcortical response inhibition nodes.
Keywords: DCM; basal ganglia; effective connectivity; inferior frontal gyrus; response inhibition.
© The Author(s) 2023. Published by Oxford University Press on behalf of West China School of Medicine/West China Hospital (WCSM/WCH) of Sichuan University.
Conflict of interest statement
K.M.K. holds the position of Editor-in-Chief and B.B. is a member of editorial board of Psychoradiology. They were blinded from the review process and making decisions on the manuscript. Disclaimer: Any opinions, findings, conclusions or recommendations expressed in this publication do not reflect the views of the Government of the Hong Kong Special Administrative Region or the Innovation and Technology Commission.
Figures


Similar articles
-
Cortical and subcortical contributions to non-motor inhibitory control: an fMRI study.Cereb Cortex. 2023 Oct 14;33(21):10909-10917. doi: 10.1093/cercor/bhad336. Cereb Cortex. 2023. PMID: 37724423
-
The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated.Neuroscience. 1994 Nov;63(2):363-79. doi: 10.1016/0306-4522(94)90536-3. Neuroscience. 1994. PMID: 7891852 Review.
-
Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study.Brain Struct Funct. 2016 Apr;221(3):1635-51. doi: 10.1007/s00429-015-0994-y. Epub 2015 Feb 1. Brain Struct Funct. 2016. PMID: 25637472 Free PMC article.
-
Altered functional connectivity of right inferior frontal gyrus subregions in bipolar disorder: a resting state fMRI study.J Affect Disord. 2020 Jul 1;272:58-65. doi: 10.1016/j.jad.2020.03.122. Epub 2020 Apr 29. J Affect Disord. 2020. PMID: 32379621
-
The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry.Brain Res Brain Res Rev. 1997 Feb;23(1-2):62-78. doi: 10.1016/s0165-0173(96)00018-5. Brain Res Brain Res Rev. 1997. PMID: 9063587 Review.
Cited by
-
A surface-based cross-sectional fMRI study on brain function differences between comorbid mild or moderate depression and insomnia.Front Neurosci. 2025 May 13;19:1554287. doi: 10.3389/fnins.2025.1554287. eCollection 2025. Front Neurosci. 2025. PMID: 40433498 Free PMC article.
-
Transcutaneous auricular vagus nerve stimulation enhanced emotional inhibitory control via increasing intrinsic prefrontal couplings.Int J Clin Health Psychol. 2024 Apr-Jun;24(2):100462. doi: 10.1016/j.ijchp.2024.100462. Epub 2024 Apr 17. Int J Clin Health Psychol. 2024. PMID: 38665809 Free PMC article.
-
Enhanced disgust generalization in obsessive-compulsive disorder is related to insula and putamen hyperactivity.Psychol Med. 2025 Apr 14;55:e116. doi: 10.1017/S0033291725000728. Psychol Med. 2025. PMID: 40223574 Free PMC article.
-
Regional and whole-brain neurofunctional alterations during pain empathic processing of physical but not affective pain in migraine patients.J Headache Pain. 2025 Jun 12;26(1):137. doi: 10.1186/s10194-025-02051-x. J Headache Pain. 2025. PMID: 40506706 Free PMC article.
-
Striatal functional connectivity associated with Sahaja Yoga meditation.Sci Rep. 2025 Apr 25;15(1):14513. doi: 10.1038/s41598-025-98256-w. Sci Rep. 2025. PMID: 40281041 Free PMC article.
References
-
- Ahissar E, Oram T (2015) Thalamic relay or cortico-thalamic processing? Old question, new answers. Cereb Cortex. 25:845–8. - PubMed
-
- Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13:266–71. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR (1991) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor,“prefrontal” and “limbic” functions. Prog Brain Res. 85:119–46. - PubMed
-
- Alexander GE, Delong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 9:357–81. - PubMed
LinkOut - more resources
Full Text Sources
