Outlining cardiac ion channel protein interactors and their signature in the human electrocardiogram
- PMID: 38666184
- PMCID: PMC11041666
- DOI: 10.1038/s44161-023-00294-y
Outlining cardiac ion channel protein interactors and their signature in the human electrocardiogram
Abstract
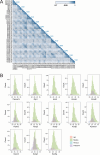
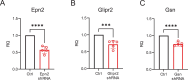
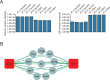
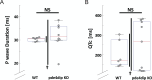
Protein-protein interactions are essential for normal cellular processes and signaling events. Defining these interaction networks is therefore crucial for understanding complex cellular functions and interpretation of disease-associated gene variants. We need to build a comprehensive picture of the interactions, their affinities and interdependencies in the specific organ to decipher hitherto poorly understood signaling mechanisms through ion channels. Here we report the experimental identification of the ensemble of protein interactors for 13 types of ion channels in murine cardiac tissue. Of these, we validated the functional importance of ten interactors on cardiac electrophysiology through genetic knockouts in zebrafish, gene silencing in mice, super-resolution microscopy and patch clamp experiments. Furthermore, we establish a computational framework to reconstruct human cardiomyocyte ion channel networks from deep proteome mapping of human heart tissue and human heart single-cell gene expression data. Finally, we integrate the ion channel interactome with human population genetics data to identify proteins that influence the electrocardiogram (ECG). We demonstrate that the combined channel network is enriched for proteins influencing the ECG, with 44% of the network proteins significantly associated with an ECG phenotype. Altogether, we define interactomes of 13 major cardiac ion channels, contextualize their relevance to human electrophysiology and validate functional roles of ten interactors, including two regulators of the sodium current (epsin-2 and gelsolin). Overall, our data provide a roadmap for our understanding of the molecular machinery that regulates cardiac electrophysiology.
Keywords: Cardiovascular biology; Data integration; Protein analysis; Proteomics.
© The Author(s) 2023.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures











References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
