Myocardial reperfusion injury exacerbation due to ALDH2 deficiency is mediated by neutrophil extracellular traps and prevented by leukotriene C4 inhibition
- PMID: 38666340
- PMCID: PMC11089336
- DOI: 10.1093/eurheartj/ehae205
Myocardial reperfusion injury exacerbation due to ALDH2 deficiency is mediated by neutrophil extracellular traps and prevented by leukotriene C4 inhibition
Abstract
Background and aims: The Glu504Lys polymorphism in the aldehyde dehydrogenase 2 (ALDH2) gene is closely associated with myocardial ischaemia/reperfusion injury (I/RI). The effects of ALDH2 on neutrophil extracellular trap (NET) formation (i.e. NETosis) during I/RI remain unknown. This study aimed to investigate the role of ALDH2 in NETosis in the pathogenesis of myocardial I/RI.
Methods: The mouse model of myocardial I/RI was constructed on wild-type, ALDH2 knockout, peptidylarginine deiminase 4 (Pad4) knockout, and ALDH2/PAD4 double knockout mice. Overall, 308 ST-elevation myocardial infarction patients after primary percutaneous coronary intervention were enrolled in the study.
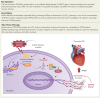
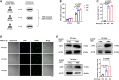
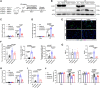
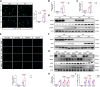
Results: Enhanced NETosis was observed in human neutrophils carrying the ALDH2 genetic mutation and ischaemic myocardium of ALDH2 knockout mice compared with controls. PAD4 knockout or treatment with NETosis-targeting drugs (GSK484, DNase1) substantially attenuated the extent of myocardial damage, particularly in ALDH2 knockout. Mechanistically, ALDH2 deficiency increased damage-associated molecular pattern release and susceptibility to NET-induced damage during myocardial I/RI. ALDH2 deficiency induced NOX2-dependent NETosis via upregulating the endoplasmic reticulum stress/microsomal glutathione S-transferase 2/leukotriene C4 (LTC4) pathway. The Food and Drug Administration-approved LTC4 receptor antagonist pranlukast ameliorated I/RI by inhibiting NETosis in both wild-type and ALDH2 knockout mice. Serum myeloperoxidase-DNA complex and LTC4 levels exhibited the predictive effect on adverse left ventricular remodelling at 6 months after primary percutaneous coronary intervention in ST-elevation myocardial infarction patients.
Conclusions: ALDH2 deficiency exacerbates myocardial I/RI by promoting NETosis via the endoplasmic reticulum stress/microsomal glutathione S-transferase 2/LTC4/NOX2 pathway. This study hints at the role of NETosis in the pathogenesis of myocardial I/RI, and pranlukast might be a potential therapeutic option for attenuating I/RI, particularly in individuals with the ALDH2 mutation.
Keywords: Aldehyde dehydrogenase 2; Leukotriene C4 receptor antagonist; Microsomal glutathione S-transferase 2; Myocardial ischaemia/reperfusion injury; NETosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

