Differential sex-dependent susceptibility to diastolic dysfunction and arrhythmia in cardiomyocytes from obese diabetic heart failure with preserved ejection fraction model
- PMID: 38666446
- PMCID: PMC12012442
- DOI: 10.1093/cvr/cvae070
Differential sex-dependent susceptibility to diastolic dysfunction and arrhythmia in cardiomyocytes from obese diabetic heart failure with preserved ejection fraction model
Abstract
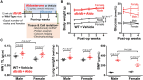
Aims: Sex differences in heart failure with preserved ejection fraction (HFpEF) are important, but key mechanisms involved are incompletely understood. While animal models can inform about sex-dependent cellular and molecular changes, many previous pre-clinical HFpEF models have failed to recapitulate sex-dependent characteristics of human HFpEF. We tested for sex differences in HFpEF using a two-hit mouse model (leptin receptor-deficient db/db mice plus aldosterone infusion for 4 weeks; db/db + Aldo).
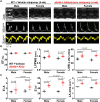
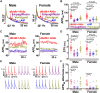
Methods and results: We performed echocardiography, electrophysiology, intracellular Ca2+ imaging, and protein analysis. Female HFpEF mice exhibited more severe diastolic dysfunction in line with increased titin N2B isoform expression and PEVK element phosphorylation and reduced troponin-I phosphorylation. Female HFpEF mice had lower BNP levels than males despite similar comorbidity burden (obesity, diabetes) and cardiac hypertrophy in both sexes. Male HFpEF mice were more susceptible to cardiac alternans. Male HFpEF cardiomyocytes (vs. female) exhibited higher diastolic [Ca2+], slower Ca2+ transient decay, reduced L-type Ca2+ current, more pronounced enhancement of the late Na+ current, and increased short-term variability of action potential duration (APD). However, male and female HFpEF myocytes showed similar downregulation of inward rectifier and transient outward K+ currents, APD prolongation, and frequency of delayed afterdepolarizations. Inhibition of Ca2+/calmodulin-dependent protein kinase II (CaMKII) reversed all pathological APD changes in HFpEF in both sexes, and empagliflozin pre-treatment mimicked these effects of CaMKII inhibition. Vericiguat had only slight benefits, and these effects were larger in HFpEF females.
Conclusion: We conclude that the db/db + Aldo pre-clinical HFpEF murine model recapitulates key sex-specific mechanisms in HFpEF and provides mechanistic insights into impaired excitation-contraction coupling and sex-dependent differential arrhythmia susceptibility in HFpEF with potential therapeutic implications. In male HFpEF myocytes, altered Ca2+ handling and electrophysiology aligned with diastolic dysfunction and arrhythmias, while worse diastolic dysfunction in females may depend more on altered myofilament properties.
Keywords: Arrhythmia; CaMKII; Diastolic dysfunction; HFpEF; Sex differences.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures





References
-
- Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 2017;97:1–37. - PubMed
-
- Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. - PubMed
-
- Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation 2020;141:540–548. - PubMed
-
- Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018;138:198–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

