Increased Vulnerability to Ferroptosis in FUS-ALS
- PMID: 38666827
- PMCID: PMC11048265
- DOI: 10.3390/biology13040215
Increased Vulnerability to Ferroptosis in FUS-ALS
Abstract
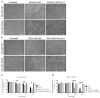
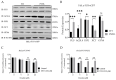
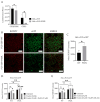
Ferroptosis, a regulated form of cell death characterized by iron-dependent lipid peroxide accumulation, plays a pivotal role in various pathological conditions, including neurodegenerative diseases. While reasonable evidence for ferroptosis exists, e.g., in Parkinson's disease or Alzheimer's disease, there are only a few reports on amyotrophic lateral sclerosis (ALS), a fast progressive and incurable neurodegenerative disease characterized by progressive motor neuron degeneration. Interestingly, initial studies have suggested that ferroptosis might be significantly involved in ALS. Key features of ferroptosis include oxidative stress, glutathione depletion, and alterations in mitochondrial morphology and function, mediated by proteins such as GPX4, xCT, ACSL4 FSP1, Nrf2, and TfR1. Induction of ferroptosis involves small molecule compounds like erastin and RSL3, which disrupt system Xc- and GPX4 activity, respectively, resulting in lipid peroxidation and cellular demise. Mutations in fused in sarcoma (FUS) are associated with familial ALS. Pathophysiological hallmarks of FUS-ALS involve mitochondrial dysfunction and oxidative damage, implicating ferroptosis as a putative cell-death pathway in motor neuron demise. However, a mechanistic understanding of ferroptosis in ALS, particularly FUS-ALS, remains limited. Here, we investigated the vulnerability to ferroptosis in FUS-ALS cell models, revealing mitochondrial disturbances and increased susceptibility to ferroptosis in cells harboring ALS-causing FUS mutations. This was accompanied by an altered expression of ferroptosis-associated proteins, particularly by a reduction in xCT expression, leading to cellular imbalance in the redox system and increased lipid peroxidation. Iron chelation with deferoxamine, as well as inhibition of the mitochondrial calcium uniporter (MCU), significantly alleviated ferroptotic cell death and lipid peroxidation. These findings suggest a link between ferroptosis and FUS-ALS, offering potential new therapeutic targets.
Keywords: amyotrophic lateral sclerosis; cell death; mitochondria; oxidative damage.
Conflict of interest statement
The authors declare that they have no competing interests related to the direct applications of this research.
Figures



References
-
- Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. - DOI - PMC - PubMed
-
- Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascon S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

