Anticandidal Activity of a Siderophore from Marine Endophyte Pseudomonas aeruginosa Mgrv7
- PMID: 38667023
- PMCID: PMC11047651
- DOI: 10.3390/antibiotics13040347
Anticandidal Activity of a Siderophore from Marine Endophyte Pseudomonas aeruginosa Mgrv7
Abstract
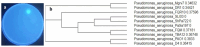
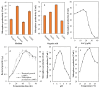
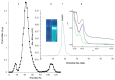
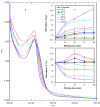
An endophytic symbiont P. aeruginosa-producing anticandidal siderophore was recovered from mangrove leaves for the first time. Production was optimal in a succinate medium supplemented with 0.4% citric acid and 15 µM iron at pH 7 and 35 °C after 60 h of fermentation. UV spectra of the acidic preparation after purification with Amberlite XAD-4 resin gave a peak at 400 nm, while the neutralized form gave a peak at 360 nm. A prominent peak with RP-HPLC was obtained at RT 18.95 min, confirming its homogeneity. It was pH stable at 5.0-9.5 and thermally stable at elevated temperatures, which encourages the possibility of its application in extreme environments. The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) against Candida spp. Were in the range of 128 µg/mL and lower. It enhanced the intracellular iron accumulation with 3.2-4.2-fold (as judged by atomic absorption spectrometry) with a subsequent increase in the intracellular antioxidative enzymes SOD and CAT. Furthermore, the malondialdehyde (MDA) concentration due to cellular lipid peroxidation increased to 3.8-fold and 7.3-fold in C. albicans and C. tropicalis, respectively. The scanning electron microscope (SEM) confirmed cellular damage in the form of roughness, malformation, and production of defensive exopolysaccharides and/or proteins after exposure to siderophore. In conclusion, this anticandidal siderophore may be a promising biocontrol, nonpolluting agent against waterborne pathogens and pathogens of the skin. It indirectly kills Candida spp. by ferroptosis and mediation of hyperaccumulation of iron rather than directly attacking the cell targets, which triggers the activation of antioxidative enzymes.
Keywords: Candida; Pseudomonas aeruginosa; antimicrobial; biocontrol; mangrove; siderophore.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Brown A.J.P., Haynes K., Gow N.A.R., Quinn J. Stress responses in Candida. In: Calderone R.A., Clancy C.J., editors. Candida and Candidiasis. 2nd ed. ASM Press; Washington, DC, USA: 2012. pp. 225–242. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

