Echinacea Reduces Antibiotics by Preventing Respiratory Infections: A Meta-Analysis (ERA-PRIMA)
- PMID: 38667040
- PMCID: PMC11047471
- DOI: 10.3390/antibiotics13040364
Echinacea Reduces Antibiotics by Preventing Respiratory Infections: A Meta-Analysis (ERA-PRIMA)
Abstract
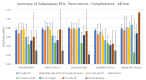
Respiratory tract infections (RTIs) are the leading cause of antibiotic prescriptions, primarily due to the risk for secondary bacterial infections. In this study, we examined whether Echinacea could reduce the need for antibiotics by preventing RTIs and their complications, and subsequently investigated its safety profile. A comprehensive search of EMBASE, PubMed, Google Scholar, Cochrane DARE and clinicaltrials.gov identified 30 clinical trials (39 comparisons) studying Echinacea for the prevention or treatment of RTIs in 5652 subjects. Echinacea significantly reduced the monthly RTI occurrence, risk ratio (RR) 0.68 (95% CI 0.61-0.77) and number of patients with ≥1 RTI, RR = 0.75 [95% CI 0.69-0.81] corresponding to an odds ratio 0.53 [95% CI 0.42-0.67]. Echinacea reduced the risk of recurrent infections (RR = 0.60; 95% CI 0.46-0.80), RTI complications (RR = 0.44; 95% CI 0.36-0.54) and the need for antibiotic therapy (RR = 0.60; 95% CI 0.39-0.93), with total antibiotic therapy days reduced by 70% (IRR = 0.29; 95% CI 0.11-0.74). Alcoholic extracts from freshly harvested Echinacea purpurea were the strongest, with an 80% reduction of antibiotic treatment days, IRR 0.21 [95% CI 0.15-0.28]. An equal number of adverse events occurred with Echinacea and control treatment. Echinacea can safely prevent RTIs and associated complications, thereby decreasing the demand for antibiotics. Relevant differences exist between Echinacea preparations.
Keywords: Echinacea; antibiotics; complications; prevention; recurrent RTIs; respiratory tract infections.
Conflict of interest statement
R.S., W.C.A. and S.L.J. received honoraria from A.Vogel AG Switzerland for consulting work. G.H. received honoraria for statistical evaluation of this meta-analysis. The other authors declare no conflicts of interest.
Figures








References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/s0140-6736(16)31678-6. Erratum in Lancet 2017, 389, e1. - DOI - PMC - PubMed
-
- WHO. 2019. [(accessed on 1 February 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

