Antibacterial Activity of Oregano (Origanum vulgare L.) Essential Oil Vapors against Microbial Contaminants of Food-Contact Surfaces
- PMID: 38667047
- PMCID: PMC11047463
- DOI: 10.3390/antibiotics13040371
Antibacterial Activity of Oregano (Origanum vulgare L.) Essential Oil Vapors against Microbial Contaminants of Food-Contact Surfaces
Abstract
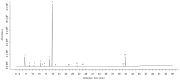
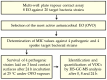
The antimicrobial effect of eight essential oils' vapors against pathogens and spoilage bacteria was assayed. Oreganum vulgare L. essential oil (OVO) showed a broad antibacterial effect, with Minimum Inhibitory Concentration (MIC) values ranging from 94 to 754 µg cm-3 air, depending on the bacterial species. Then, gaseous OVO was used for the treatment of stainless steel, polypropylene, and glass surfaces contaminated with four bacterial pathogens at 6-7 log cfu coupon-1. No viable cells were found after OVO treatment on all food-contact surfaces contaminated with all pathogens, with the exception of Sta. aureus DSM 799 on the glass surface. The antimicrobial activity of OVO after the addition of beef extract as a soiling agent reduced the Sta. aureus DSM 799 viable cell count by more than 5 log cfu coupon-1 on polypropylene and glass, while no viable cells were found in the case of stainless steel. HS-GC-MS analysis of the headspace of the boxes used for the antibacterial assay revealed 14 different volatile compounds with α-Pinene (62-63%), and p-Cymene (21%) as the main terpenes. In conclusion, gaseous OVO could be used for the microbial decontamination of food-contact surfaces, although its efficacy needs to be evaluated since it depends on several parameters such as target microorganisms, food-contact material, temperature, time of contact, and relative humidity.
Keywords: HS-GC-MS analysis; abiotic surfaces; cross-contamination; foodborne pathogens; natural antimicrobials; oregano essential oil.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity.Curr Pharm Des. 2019;25(16):1809-1816. doi: 10.2174/1381612825666190702095612. Curr Pharm Des. 2019. PMID: 31267860
-
Antioxidant and antimicrobial effect of sodium alginate nanoemulsion coating enriched with oregano essential oil (Origanum vulgare L.) and Trachyspermum ammi oil (Carum cupticum) on food pathogenic bacteria.Food Sci Nutr. 2024 Feb 16;12(4):2985-2997. doi: 10.1002/fsn3.3979. eCollection 2024 Apr. Food Sci Nutr. 2024. PMID: 38628174 Free PMC article.
-
Inhibitory effects of UV treatment and a combination of UV and dry heat against pathogens on stainless steel and polypropylene surfaces.J Food Sci. 2012 Jan;77(1):M61-4. doi: 10.1111/j.1750-3841.2011.02476.x. Epub 2011 Dec 2. J Food Sci. 2012. PMID: 22132742
-
Chemical constituent, minimal inhibitory concentration, and antimicrobial efficiency of essential oil from oreganum vulgare against Enterococcus faecalis: An in vitro study.J Conserv Dent. 2019 Nov-Dec;22(6):538-543. doi: 10.4103/JCD.JCD_80_19. Epub 2020 Aug 20. J Conserv Dent. 2019. PMID: 33088061 Free PMC article.
-
Antibacterial activity of oregano essential oils against Streptococcus mutans in vitro and analysis of active components.BMC Complement Med Ther. 2023 Feb 21;23(1):61. doi: 10.1186/s12906-023-03890-4. BMC Complement Med Ther. 2023. PMID: 36810055 Free PMC article.
Cited by
-
Application of Plant Antimicrobials in the Food Sector: Where Do We Stand?Foods. 2024 Jul 15;13(14):2222. doi: 10.3390/foods13142222. Foods. 2024. PMID: 39063306 Free PMC article.
-
Synergistic Antibacterial Effects of Plant Extracts and Essential Oils Against Drug-Resistant Bacteria of Clinical Interest.Pathogens. 2025 Apr 4;14(4):348. doi: 10.3390/pathogens14040348. Pathogens. 2025. PMID: 40333114 Free PMC article.
-
Essential oils from Lamiaceae plants effectively control Colletotrichum gloeosporioides, Elsinoë ampelina and Phytophthora infestans.Braz J Microbiol. 2025 Jun;56(2):975-989. doi: 10.1007/s42770-024-01607-4. Epub 2025 Feb 11. Braz J Microbiol. 2025. PMID: 39932661
-
Predictive Modeling for Inactivation of Escherichia coli Biofilm with Combined Treatment of Thermosonication and Organic Acid on Polystyrene Surface.Foods. 2024 Dec 11;13(24):4002. doi: 10.3390/foods13244002. Foods. 2024. PMID: 39766943 Free PMC article.
-
Three-Dimensional Structure of Biofilm Formed on Glass Surfaces Revealed Using Scanning Ion Conductance Microscopy Combined with Confocal Laser Scanning Microscopy.Microorganisms. 2025 Jul 30;13(8):1779. doi: 10.3390/microorganisms13081779. Microorganisms. 2025. PMID: 40871283 Free PMC article.
References
-
- Pinto L., Cefola M., Bonifacio M., Cometa S., Bocchino C., Pace B., De Giglio E., Palumbo M., Sada A., Logrieco A., et al. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021;336:127590. doi: 10.1016/j.foodchem.2020.127590. - DOI - PubMed
-
- Iseppi R., Tardugno R., Brighenti V., Benvenuti S., Sabia C., Pellati F., Messi P. Phytochemical Composition and In Vitro Antimicrobial Activity of Essential Oils from the Lamiaceae Family against Streptococcus agalactiae and Candida albicans Biofilms. Antibiotics. 2020;9:592. doi: 10.3390/antibiotics9090592. - DOI - PMC - PubMed
-
- Khammassi M., Ayed R.B., Loupasaki S., Amri I., Hanana M., Hamrouni L., Jamoussi B., Khaldi A. Chemical diversity of wild fennel essential oils (Foeniculum vulgare Mill.): A source of antimicrobial and antioxidant activities. South Afr. J. Bot. 2023;153:136–146. doi: 10.1016/j.sajb.2022.12.022. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

