No Sequestration of Commonly Used Anti-Infectives in the Extracorporeal Membrane Oxygenation (ECMO) Circuit-An Ex Vivo Study
- PMID: 38667049
- PMCID: PMC11047533
- DOI: 10.3390/antibiotics13040373
No Sequestration of Commonly Used Anti-Infectives in the Extracorporeal Membrane Oxygenation (ECMO) Circuit-An Ex Vivo Study
Abstract
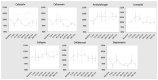
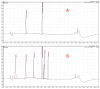
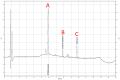
Patients undergoing extracorporeal membrane oxygenation (ECMO) often require therapy with anti-infective drugs. The pharmacokinetics of these drugs may be altered during ECMO treatment due to pathophysiological changes in the drug metabolism of the critically ill and/or the ECMO therapy itself. This study investigates the latter aspect for commonly used anti-infective drugs in an ex vivo setting. A fully functional ECMO device circulated an albumin-electrolyte solution through the ECMO tubes and oxygenator. The antibiotic agents cefazolin, cefuroxim, cefepime, cefiderocol, linezolid and daptomycin and the antifungal agent anidulafungin were added. Blood samples were taken over a period of four hours and drug concentrations were measured via high-pressure liquid chromatography (HPLC) with UV detection. Subsequently, the study analyzed the time course of anti-infective concentrations. The results showed no significant changes in the concentration of any tested anti-infectives throughout the study period. This ex vivo study demonstrates that the ECMO device itself has no impact on the concentration of commonly used anti-infectives. These findings suggest that ECMO therapy does not contribute to alterations in the concentrations of anti-infective medications in severely ill patients.
Keywords: ECMO; antibiotics; critical illness; pharmacokinetics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Cefiderocol is Not Sequestered in an Ex Vivo Extracorporeal Membrane Oxygenation (ECMO) Circuit.Eur J Drug Metab Pharmacokinet. 2023 Jul;48(4):437-441. doi: 10.1007/s13318-023-00840-w. Epub 2023 Jun 23. Eur J Drug Metab Pharmacokinet. 2023. PMID: 37351777
-
Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature.Clin Ther. 2016 Sep;38(9):1976-94. doi: 10.1016/j.clinthera.2016.07.169. Epub 2016 Aug 21. Clin Ther. 2016. PMID: 27553752 Free PMC article. Review.
-
Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients.J Clin Pharm Ther. 2017 Dec;42(6):661-671. doi: 10.1111/jcpt.12636. Epub 2017 Sep 25. J Clin Pharm Ther. 2017. PMID: 28948652 Review.
-
Ex Vivo Model to Decipher the Impact of Extracorporeal Membrane Oxygenation on Beta-lactam Degradation Kinetics.Ther Drug Monit. 2017 Apr;39(2):180-184. doi: 10.1097/FTD.0000000000000369. Ther Drug Monit. 2017. PMID: 28033162
-
Evaluation of Altered Drug Pharmacokinetics in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation.Pharmacotherapy. 2017 Feb;37(2):221-235. doi: 10.1002/phar.1882. Epub 2017 Feb 3. Pharmacotherapy. 2017. PMID: 27931091 Review.
Cited by
-
Cefiderocol pharmacokinetics in critically ill patients undergoing ECMO support.Crit Care. 2024 Oct 18;28(1):337. doi: 10.1186/s13054-024-05126-4. Crit Care. 2024. PMID: 39425201 Free PMC article. No abstract available.
-
Individual target pharmacokinetic/pharmacodynamic attainment rates among cefepime-treated patients admitted to the ICU with hospital-acquired pneumonia with and without ECMO.Antimicrob Agents Chemother. 2025 Jun 4;69(6):e0010225. doi: 10.1128/aac.00102-25. Epub 2025 May 15. Antimicrob Agents Chemother. 2025. PMID: 40372025 Free PMC article.
References
-
- Roberts J.A., Abdul-Aziz M.H., Lipman J., Mouton J.W., Vinks A.A., Felton T.W., Hope W.W., Farkas A., Neely M.N., Schentag J.J., et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. - DOI - PMC - PubMed
-
- Shekar K., Roberts J.A., Smith M.T., Fung Y.L., Fraser J.F. The ECMO PK Project: An Incremental Research Approach to Advance Understanding of the Pharmacokinetic Alterations and Improve Patient Outcomes during Extracorporeal Membrane Oxygenation. BMC Anesthesiol. 2013;13:7. doi: 10.1186/1471-2253-13-7. - DOI - PMC - PubMed
-
- Duceppe M.A., Kanji S., Do A.T., Ruo N., Cavayas Y.A., Albert M., Robert-Halabi M., Zavalkoff S., Dupont P., Samoukovic G., et al. Pharmacokinetics of Commonly Used Antimicrobials in Critically Ill Adults During Extracorporeal Membrane Oxygenation: A Systematic Review. Drugs. 2021;81:1307–1329. doi: 10.1007/s40265-021-01557-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

