Nanocomposite Methacrylated Silk Fibroin-Based Scaffolds for Bone Tissue Engineering
- PMID: 38667229
- PMCID: PMC11048339
- DOI: 10.3390/biomimetics9040218
Nanocomposite Methacrylated Silk Fibroin-Based Scaffolds for Bone Tissue Engineering
Abstract
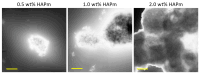
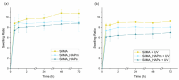
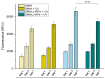
The treatment of bone defects is a clinical challenge. Bone tissue engineering is gaining interest as an alternative to current treatments, with the development of 3D porous structures (scaffolds) helpful in promoting bone regeneration by ensuring temporary functional support. In this work, methacrylated silk fibroin (SilMA) sponges were investigated as scaffolds for bone tissue engineering by exploiting the combination of physical (induced by NaCl salt during particulate leaching) and chemical crosslinking (induced by UV-light exposure) techniques. A biomimetic approach was adopted to better simulate the extracellular matrix of the bone by introducing either natural (mussel shell-derived) or synthetic-origin hydroxyapatite nanoparticles into the SilMA sponges. The obtained materials were characterized in terms of pore size, water absorption capability and mechanical properties to understand both the effect of the inclusion of the two different types of nanoparticles and the effect of the photocrosslinking. Moreover, the SilMA sponges were tested for their bioactivity and suitability for bone tissue engineering purposes by using osteosarcoma cells, studying their metabolism by an AlamarBlue assay and their morphology by scanning electron microscopy. Results indicate that photocrosslinking helps in obtaining more regular structures with bimodal pore size distributions and in enhancing the stability of the constructs in water. Moreover, the addition of naturally derived hydroxyapatite was observed to be more effective at activating osteosarcoma cell metabolism than synthetic hydroxyapatite, showing a statistically significant difference in the AlamarBlue measurement on day 7 after seeding. The methacrylated silk fibroin/hydroxyapatite nanocomposite sponges developed in this work were found to be promising tools for targeting bone regeneration with a sustainable approach.
Keywords: bone tissue engineering; hydroxyapatite; methacrylated silk fibroin; scaffold.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite-tussah silk fibroin nanoparticles for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2016 Jan 1;58:342-51. doi: 10.1016/j.msec.2015.08.046. Epub 2015 Sep 1. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26478319
-
Precipitation of hydroxyapatite on electrospun polycaprolactone/aloe vera/silk fibroin nanofibrous scaffolds for bone tissue engineering.J Biomater Appl. 2014 Jul;29(1):46-58. doi: 10.1177/0885328213513934. Epub 2013 Nov 27. J Biomater Appl. 2014. PMID: 24287981
-
Hydroxyapatite reinforced inherent RGD containing silk fibroin composite scaffolds: Promising platform for bone tissue engineering.Nanomedicine. 2017 Jul;13(5):1745-1759. doi: 10.1016/j.nano.2017.02.016. Epub 2017 Mar 8. Nanomedicine. 2017. PMID: 28285159
-
Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110698. doi: 10.1016/j.msec.2020.110698. Epub 2020 Jan 29. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204012 Free PMC article. Review.
-
Silk Fibroin Combined with Electrospinning as a Promising Strategy for Tissue Regeneration.Macromol Biosci. 2023 Feb;23(2):e2200380. doi: 10.1002/mabi.202200380. Epub 2022 Dec 7. Macromol Biosci. 2023. PMID: 36409150 Review.
Cited by
-
Rapid neuralized and vascularized osteogenesis in infected bone defect using biomimetic biomineralized and antibacterial hydrogels.Front Bioeng Biotechnol. 2025 May 21;13:1611639. doi: 10.3389/fbioe.2025.1611639. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40470505 Free PMC article.
-
Biomimetic Three-Dimensional (3D) Scaffolds from Sustainable Biomaterials: Innovative Green Medicine Approach to Bone Regeneration.J Funct Biomater. 2025 Jun 29;16(7):238. doi: 10.3390/jfb16070238. J Funct Biomater. 2025. PMID: 40710453 Free PMC article. Review.
-
Interpenetrating network hydrogel-loaded embryonic stem cell-derived endocardial cells improves cardiac function after myocardial infarction.J Transl Med. 2025 May 30;23(1):603. doi: 10.1186/s12967-025-06603-2. J Transl Med. 2025. PMID: 40448180 Free PMC article.
-
Future-Oriented Biomaterials Based on Natural Polymer Resources: Characteristics, Application Innovations, and Development Trends.Int J Mol Sci. 2025 Jun 9;26(12):5518. doi: 10.3390/ijms26125518. Int J Mol Sci. 2025. PMID: 40564982 Free PMC article. Review.
References
-
- Kengelbach-Weigand A., Thielen C., Bäuerle T., Götzl R., Gerber T., Körner C., Beier J.P., Horch R.E., Boos A.M. Personalized Medicine for Reconstruction of Critical-Size Bone Defects—A Translational Approach with Customizable Vascularized Bone Tissue. NPJ Regen. Med. 2021;6:49. doi: 10.1038/s41536-021-00158-8. - DOI - PMC - PubMed
-
- Kiernan C., Knuth C., Farrell E. Chapter 6—Endochondral Ossification: Recapitulating Bone Development for Bone Defect Repair. In: Stoddart M.J., Craft A.M., Pattappa G., Gardner O.F.W., editors. Developmental Biology and Musculoskeletal Tissue Engineering. Academic Press; Boston, MA, USA: 2018. pp. 125–148.
Grants and funding
LinkOut - more resources
Full Text Sources

