Protecting Orthopaedic Implants from Infection: Antimicrobial Peptide Mel4 Is Non-Toxic to Bone Cells and Reduces Bacterial Colonisation When Bound to Plasma Ion-Implanted 3D-Printed PAEK Polymers
- PMID: 38667271
- PMCID: PMC11049013
- DOI: 10.3390/cells13080656
Protecting Orthopaedic Implants from Infection: Antimicrobial Peptide Mel4 Is Non-Toxic to Bone Cells and Reduces Bacterial Colonisation When Bound to Plasma Ion-Implanted 3D-Printed PAEK Polymers
Abstract
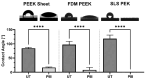
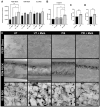
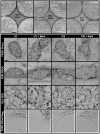
Even with the best infection control protocols in place, the risk of a hospital-acquired infection of the surface of an implanted device remains significant. A bacterial biofilm can form and has the potential to escape the host immune system and develop resistance to conventional antibiotics, ultimately causing the implant to fail, seriously impacting patient well-being. Here, we demonstrate a 4 log reduction in the infection rate by the common pathogen S. aureus of 3D-printed polyaryl ether ketone (PAEK) polymeric surfaces by covalently binding the antimicrobial peptide Mel4 to the surface using plasma immersion ion implantation (PIII) treatment. The surfaces with added texture created by 3D-printed processes such as fused deposition-modelled polyether ether ketone (PEEK) and selective laser-sintered polyether ketone (PEK) can be equally well protected as conventionally manufactured materials. Unbound Mel4 in solution at relevant concentrations is non-cytotoxic to osteoblastic cell line Saos-2. Mel4 in combination with PIII aids Saos-2 cells to attach to the surface, increasing the adhesion by 88% compared to untreated materials without Mel4. A reduction in mineralisation on the Mel4-containing surfaces relative to surfaces without peptide was found, attributed to the acellular portion of mineral deposition.
Keywords: 3D printing; antimicrobial peptide; biofilm; filament deposition modelling; infection prevention; orthopaedic implant; plasma immersion ion implantation; polyether ether ketone; polyether ketone; selective laser sintering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

