Development and Characterisation of a New Patient-Derived Xenograft Model of AR-Negative Metastatic Castration-Resistant Prostate Cancer
- PMID: 38667288
- PMCID: PMC11049137
- DOI: 10.3390/cells13080673
Development and Characterisation of a New Patient-Derived Xenograft Model of AR-Negative Metastatic Castration-Resistant Prostate Cancer
Abstract
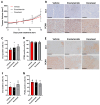
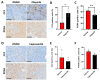
As the treatment landscape for prostate cancer gradually evolves, the frequency of treatment-induced neuroendocrine prostate cancer (NEPC) and double-negative prostate cancer (DNPC) that is deficient for androgen receptor (AR) and neuroendocrine (NE) markers has increased. These prostate cancer subtypes are typically refractory to AR-directed therapies and exhibit poor clinical outcomes. Only a small range of NEPC/DNPC models exist, limiting our molecular understanding of this disease and hindering our ability to perform preclinical trials exploring novel therapies to treat NEPC/DNPC that are urgently needed in the clinic. Here, we report the development of the CU-PC01 PDX model that represents AR-negative mCRPC with PTEN/RB/PSMA loss and CTNN1B/TP53/BRCA2 genetic variants. The CU-PC01 model lacks classic NE markers, with only focal and/or weak expression of chromogranin A, INSM1 and CD56. Collectively, these findings are most consistent with a DNPC phenotype. Ex vivo and in vivo preclinical studies revealed that CU-PC01 PDX tumours are resistant to mCRPC standard-of-care treatments enzalutamide and docetaxel, mirroring the donor patient's treatment response. Furthermore, short-term CU-PC01 tumour explant cultures indicate this model is initially sensitive to PARP inhibition with olaparib. Thus, the CU-PC01 PDX model provides a valuable opportunity to study AR-negative mCRPC biology and to discover new treatment avenues for this hard-to-treat disease.
Keywords: androgen receptor (AR); castration-resistant prostate cancer (CRPC); double-negative prostate cancer (DNPC); neuroendocrine (NE); patient-derived xenograft (PDX).
Conflict of interest statement
H.B.P. has two active open innovation projects with AstraZeneca to preclinically explore AKT and PARP inhibitors in prostate cancer. M.C.H. served as a paid consultant/received honoraria from Pfizer and has received research funding from Merck, Novartis, Genentech, Promicell, and Bristol Myers Squibb. P.S.N. served as a paid consultant and received honoraria from Pfizer, Bristol Myers Squibb, and Merck and received research support from Janssen for work unrelated to the present studies. J.N.S. reports personal fees and non-financial support from Janssen Oncology, and personal fees from Astellas, outside the submitted work within the past 5 years. J.N.S. has also received personal fees from AstraZeneca that have an ongoing clinical development program for an AKT inhibitor. All other authors declare no conflicts of interest.
Figures




References
-
- Oh W.K., Miao R., Vekeman F., Sung J., Cheng W.Y., Gauthier-Loiselle M., Dhawan R., Duh M.S. Real-world Characteristics and Outcomes of Patients With Metastatic Castration-resistant Prostate Cancer Receiving Chemotherapy Versus Androgen Receptor-targeted Therapy After Failure of First-line Androgen Receptor-targeted Therapy in the Community Setting. Clin. Genitourin. Cancer. 2017;16:50–57. doi: 10.1016/j.clgc.2017.06.004. - DOI - PubMed
-
- Bluemn E.G., Coleman I.M., Lucas J.M., Coleman R.T., Hernandez-Lopez S., Tharakan R., Bianchi-Frias D., Dumpit R.F., Kaipainen A., Corella A.N., et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell. 2017;32:474–489.e476. doi: 10.1016/j.ccell.2017.09.003. - DOI - PMC - PubMed
-
- Labrecque M.P., Coleman I.M., Brown L.G., True L.D., Kollath L., Lakely B., Nguyen H.M., Yang Y.C., da Costa R.M.G., Kaipainen A., et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Investig. 2019;129:4492–4505. doi: 10.1172/JCI128212. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

