From Hematopoietic Stem Cells to Platelets: Unifying Differentiation Pathways Identified by Lineage Tracing Mouse Models
- PMID: 38667319
- PMCID: PMC11048769
- DOI: 10.3390/cells13080704
From Hematopoietic Stem Cells to Platelets: Unifying Differentiation Pathways Identified by Lineage Tracing Mouse Models
Abstract
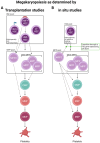
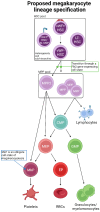
Platelets are the terminal progeny of megakaryocytes, primarily produced in the bone marrow, and play critical roles in blood homeostasis, clotting, and wound healing. Traditionally, megakaryocytes and platelets are thought to arise from multipotent hematopoietic stem cells (HSCs) via multiple discrete progenitor populations with successive, lineage-restricting differentiation steps. However, this view has recently been challenged by studies suggesting that (1) some HSC clones are biased and/or restricted to the platelet lineage, (2) not all platelet generation follows the "canonical" megakaryocytic differentiation path of hematopoiesis, and (3) platelet output is the default program of steady-state hematopoiesis. Here, we specifically investigate the evidence that in vivo lineage tracing studies provide for the route(s) of platelet generation and investigate the involvement of various intermediate progenitor cell populations. We further identify the challenges that need to be overcome that are required to determine the presence, role, and kinetics of these possible alternate pathways.
Keywords: hematopoietic stem cell (HSC); lineage tracing; megakaryocyte progenitor (MkP); megakaryopoiesis; platelet; thrombopoiesis; transplantation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures