Prospects for Controlling Hepatitis B Globally
- PMID: 38668246
- PMCID: PMC11054959
- DOI: 10.3390/pathogens13040291
Prospects for Controlling Hepatitis B Globally
Abstract
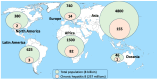
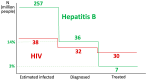
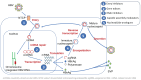
Infection with the hepatitis B virus (HBV) is highly prevalent globally. Over 250 million people suffer from chronic hepatitis B, and more than 800,000 patients die each year due to hepatitis B complications, including liver cancer. Although protective HBV vaccines are recommended for all newborns, global coverage is suboptimal. In adults, sexual transmission is by far the most frequent route of contagion. The WHO estimates that 1.5 million new HBV infections occur annually. Oral nucleos(t)ide analogues entecavir and tenofovir are the most frequent antivirals prescribed as HBV therapy. Almost all patients adherent to the medication achieve undetectable plasma viremia beyond 6 months of monotherapy. However, less than 5% achieve anti-HBs seroconversion, and viral rebound occurs following drug discontinuation. Therefore, nucleos(t)ide analogues need to be lifelong. New long-acting formulations of tenofovir and entecavir are being developed that will maximize treatment benefit and overcome adherence barriers. Furthermore, new antiviral agents are in development, including entry inhibitors, capside assembly modulators, and RNA interference molecules. The use of combination therapy pursues a functional HBV cure, meaning it is negative for both circulating HBV-DNA and HBsAg. Even when this goal is achieved, the cccDNA reservoir within infected hepatocytes remains a signal of past infection, and HBV can reactivate under immune suppression. Therefore, new gene therapies, including gene editing, are eagerly being pursued to silence or definitively disrupt HBV genomes within infected hepatocytes and, in this way, ultimately cure hepatitis B. At this time, three actions can be taken to push HBV eradication globally: (1) expand universal newborn HBV vaccination; (2) perform once-in-life testing of all adults to identify susceptible HBV persons that could be vaccinated (or re-vaccinated) and unveil asymptomatic carriers that could benefit from treatment; and (3) provide earlier antiviral therapy to chronic HBV carriers, as being aviremic reduces the risk of both clinical progression and transmission.
Keywords: bulevirtide; chronic hepatitis B; entecavir; gene editing; hepatitis delta; long-acting antivirals; tenofovir.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the collection, analysis, or interpretation of published literature, in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- WHO Hepatitis B. 2023. [(accessed on 3 March 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous