Dynamic Localization of Paraspeckle Components under Osmotic Stress
- PMID: 38668381
- PMCID: PMC11053584
- DOI: 10.3390/ncrna10020023
Dynamic Localization of Paraspeckle Components under Osmotic Stress
Abstract
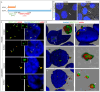
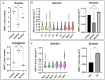
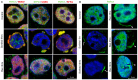
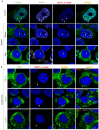
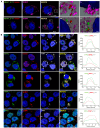
Paraspeckles are nuclear condensates formed by NEAT1_2 lncRNA and different RNA-binding proteins. In general, these membraneless organelles function in the regulation of gene expression and translation and in miRNA processing, and in doing this, they regulate cellular homeostasis and mediate pro-survival in the cell. Despite evidence showing the importance of paraspeckles in the stress response, the dynamics of paraspeckles and their components under conditions of osmotic stress remain unknown. We exposed HEK293T cells to sorbitol and examined NEAT1_2 expression using real-time PCR. Localization and quantification of the main paraspeckle components, NEAT1_2, PSPC1, NONO, and SFPQ, in different cellular compartments was performed using smFISH and immunofluorescence. Our findings showed a significant decrease in total NEAT1_2 expression in cells after osmotic stress. Sorbitol shifted the subcellular localization of NEAT1_2, PSPC1, NONO, and SFPQ from the nucleus to the cytoplasm and decreased the number and size of NEAT1_2 foci in the nucleus. PSPC1 formed immunoreactive cytoplasmic fibrils under conditions of osmotic stress, which slowly disassembled under recovery. Our study deepens the paraspeckle dynamics in response to stress, suggesting a novel role for NEAT1_2 in the cytoplasm in osmotic stress and physiological conditions.
Keywords: NEAT1_2; cytoplasmic aggregates; membraneless organelles; osmotic stress; paraspeckle proteins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Hirose T., Virnicchi G., Tanigawa A., Naganuma T., Li R., Kimura H., Yokoi T., Nakagawa S., Bénard M., Fox A.H., et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. Cell. 2014;25:169–183. doi: 10.1091/mbc.e13-09-0558. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

